1.9: Solutions to Selected Problems
- Page ID
- 195476
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)Exercise 1.1.1:
Reactions that go down in energy will proceed. Reactions that go up in energy will not proceed. If the reaction profile is higher on the left (reactant side) than the right, the reaction will go forward and it will form products. If the reverse is true, the reaction presumably will not occur.
Exercise 1.2.1:
b) Because there are two carbons in ethane, one molecule of ethane will give rise to two molecules of CO2.
c) Because there are six hydrogens in ethane, one molecule of ethane will give rise to three molecules of H2O.
d) In order to make two molecules of carbon dioxide (four oxygen atoms) and three water molecules (three oxygen atoms), we would need seven oxygen atoms total. Since oxygen molecules contain pairs of oxygen atoms, we would only need 3.5 oxygen molecules.
In principle, three and a half molecules is a problem. Where are we going to get a half of a molecule? In practice, it's nothing to worry about. We can't really do reactions with single molecules anyway. We are always working with vast numbers of molecules, but we have to make sure we keep them in the right ratio. Instead of using one molecule of ethane, we might use one billion ethane molecules, and 3.5 billion oxygen molecules.
a) The reaction is given with structures below:
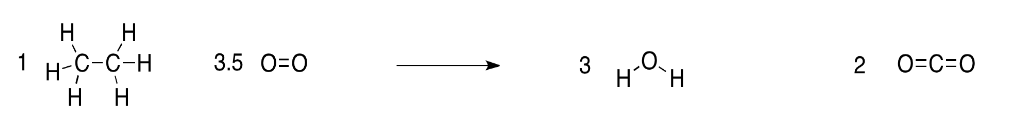
e) The energy requirements are laid out in the following table. Overall, the reaction releases 375.5 kcal per mol of ethane burned. The negative sign in the table is often used to denote that this is excess energy released (whereas a positive sign would indicate that energy as consumed overall).
| Bond Breaking | Costs (kcal/mol) | Sum of Cost | Bond Making | Releases (kcal/mol) | Sum of Release | Overall (kcal/mol) | |
| 6 x C-H | 6 * 99 | 594 | 6 x O-H | 6 * 111 | 666 | ||
| 3.5 x O=O | 3.5 * 119 | 416.5 | 4 x C=O | 4 * 180 | 720 | ||
| 1 x C-C | 83 | 83 | |||||
| total | breaking: | 1093.5 | making: | 1386 | -292.5 |
That's more energy than was produced from a molecule of methane (-170 kcal/mol).
Exercise 1.3.1:
Entropy is higher if the energy is partitioned into more states. For example, in question (b), the same amount of energy is distributed into three states on the left hand side and only two states on the right. Entropy is higher in the left hand example than the right in that case.
Exercise 1.3.2:
Examples of "states" into which energy can be partitioned include molecular vibrational, rotational and translational states (which, loosely speaking, correspond to wiggling, spinning and zipping around). Entropy is higher if energy is distributed into more of these states. That might include a greater range of vibrational or rotational states used in (a) and (c), or similar states employed in a greater number of molecules in (b).
Exercise 1.3.3:
One general observation about internal entropy is that it increases if the number of molecules increases during a reaction and decreases if the number of molecules decreases during a reaction. It's just a matter of counting how many things on the left get turned into how many things on the right. For example, in question (a), one molecule produces two new molecules in the decarboxylation reaction, so the reaction is entropically favored.
Exercise 1.4.1:
The expression ΔG = ΔH - TΔS includes both an enthalpy contribution and an enthalpy contribution and balances them against each other. However, the effect of entropy is multiplied by the temperature. The greater the temperature, the greater will be the influence of entropy (and therefore the smaller the influence of enthalpy). The lower the temperature, the smaller will be the influence of entropy (and therefore the greater the influence of enthalpy).
Exercise 1.4.2:
See problem 1.1.1
Exercise 1.5.1:
The removal of any item produced on the right side of the reaction will shift the reaction to the right in order to restore equilibrium. On the other hand, adding any more of any of the items on the right will shift the reaction to the left.
Items on the left side will work in the opposite way. Adding more of anything on the left will shift the reaction to the right, to use up the newly added materials. Removing anything from the left will shift the reaction further left, to replace the items that were removed.
- The amount of water increases, moving the reaction to the right. More products are made.
- The amount of energy increases, moving the reaction to the left. Fewer products are made.
- The amount of carbon dioxide decreases, shifting the reaction to the right. More products are made.
- The amount of energy decreases, shifting the reaction to the right. More products are made.
- The amount of carbon monoxide decreases, shifting the reaction to the left. Fewer products are made.
Exercise 1.5.2:
- The amount of energy increases, moving the reaction to the left. Fewer products are made.
- The amount of hydrogen chloride increases, shifting the reaction to the right. More products are made.
- The amount of acetylene decreases, shifting the reaction to the left. Fewer products are made.
- The amount of energy decreases, shifting the reaction to the right. More products are made.
- This question does not follow the pattern. However, because the products and reactants are all gases, we can think about the effect they would have on pressure if the reaction moved one way or the other. Because fewer gas molecules are produced on the right than the left, pressure would decrease on going from left to right (and increase on going from right to left). Thus, we can pencil in "pressure" as an item on the left side of the reaction. That means increasing pressure will shift the reaction to the right, making more products.
Exercise 1.5.3:
- The amount of energy increases, shifting the reaction to the right. More products are made.
- The amount of energy decreases, moving the reaction to the left. Fewer products are made.
- The amount of phosphate increases, shifting the reaction to the right. More products are made.
Exercise 1.5.4:
- The nitric acid would build up in the water, and the NO gas would build up, until equilibrium is reached. The nitric acid in the water would be limited by that equilibrium point.
- Periodically removing the nitric acid solution and adding fresh water would help to shift the reaction further to the right, although the eventual buildup of NO gas might prevent the reaction from shifting too far.
- A constant source of both water and nitrogen dioxide (nitric oxide) would help to push the reaction to the right. Although allowing gases to vent would limit the amount of nitrogen dioxide in the system, it would also prevent a buildup of nitrogen monoxide (nitrous oxide), which would otherwise push the reaction to the left, eventually.
Exercise 1.6.1:
The exponent is the number of times the base number is multiplied by itself. For example, 103 = 10 x 10 x 10. The higher the exponent, the larger the resulting mathematical product.
The same is true with the magnitude of a negative exponent, but the negative sign means that we are dealing with the inverse of the base number. For example, \(10^{-2} = \frac{1}{10} \times \frac{1}{10} = \frac{1}{(10 \times 10)}\)
Exercise 1.6.2:
The greater (and more positive) the free energy change, the smaller the equilibrium constant.
However, the greater (and more negative) the free energy change, the larger the equilibrium constant.
Equilibrium constants, from largest to smallest, would have associated free energies as follows:
(large K) big, negative ΔG > small, negative ΔG > small, positive ΔG > large, positive ΔG (small K)
Exercise 1.6.3:
This is just an algorithm problem, but don't forget to convert kcal to cal.
For example, in (a), \(K = e^{-\frac{3000 cal \: mol^{-1}}{1.986 cal \: K^{-1} \: mol^{-1} \times 300K}} = e^{-5.035} = 0.0065 \)
Exercise 1.6.4:
Remember, the closer K gets to 1, the closer the system gets to an equal mix of reactants and products. That's a slight approximation, because the value of K when there is an equal amount of reactants and products may be more or less than one depending on how many molecules (or moles) of each species are involved in the reaction.
Exercise 1.6.5:
There are a couple of reasons, but one involves the enthalpy requirement compared to the available energy. Temperature is an index of how much energy is available in the surroundings. The more energy is available from the surroundings, the more likely energy can be supplied to overcome a deficit in enthalpy, for either the forward or the reverse reaction. Thus at high temperature, the equilibrium is just as likely to sit on the high energy side of the reaction as it is on the low energy side.
Another way of looking at things is that the external entropy change is relatively small at high temperature, because the additional distribution of energy resulting from the reaction is very small compared to the pre-existing distribution of external energy when there is already a lot of energy in the surroundings. That leaves only the internal entropy change to govern the equilibrium.
Exercise 1.7.1:
a) K = 1020-9 = 1011
Exercise 1.7.2:
a) K = 1017-19 = 10-2
Exercise 1.8.1:
a) We could write the equations for the reactions, including the energy change involved:
\[K + 0.5 Cl_{2} \rightarrow KCl + 104 \frac{kcal}{mol} \nonumber\]
There is a 0.5 in front of the Cl2. Although chlorine is diatomic in its elemental state, we only need half the number of Cl2 molecules as we need potassium atoms if we are to form potassium chloride.
\[K + 0.5 Cl_{2} + O_{2} \rightarrow KClO_{2} + 95 \frac{kcal}{mol} \nonumber\]
In both cases, the heat of formation is negative, so we are writing that energy as a product of the reaction.
If we write the first reaction in reverse,
\[104 \frac{kcal}{mol} + KCl \rightarrow K + 0.5 Cl_{2} \nonumber\]
then we are saying that the reverse reaction would require the input of energy.
We will combine those two equations by adding them together:
\[K + 0.5Cl_{2} + O_{2} \rightarrow KClO_{2} + 95 \frac{kcal}{mol} \nonumber\]
\[104 \frac{kcal}{mol} + KCl \rightarrow K + 0.5Cl_{2} \nonumber\]
Sum:
\[104 \frac{kcal}{mol} + KCl + K + 0.5Cl_{2} + O_{2} \rightarrow K + 0.5Cl_{2} + KClO_{2} _ 95 \frac{kcal}{mol} \nonumber\]
Simplifying (the 0.5 chlorine molecules and potassium atoms appear on both sides, so they cancel):
\[104 \frac{kcal}{mol} + KCl + O_{2} \rightarrow KClO_{2} + 95 \frac{kcal}{mol} \nonumber\]
Now we use more algebra and combine the numerical part together:
\[104 \frac{kcal}{mol} -95 \frac{kcal}{mol} + KCl + O_{2} \rightarrow KClO_{2} + 95 \frac{kcal}{mol} - 95 \frac{kcal}{mol} \nonumber\]
Which leaves:
\[9 \frac{kcal}{mol} + KCl + O_{2} \rightarrow KClO_{2} \nonumber\]
The energy is added on the left. It is needed for the reaction. The heat of reaction, ΔHrxn = + 9 kcal/mol. It's an endothermic reaction.


