14.20: Solutions to Selected Problems
- Page ID
- 194555
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)With contributions from Nicholas Jones and Kate Graham, College of Saint Benedict / Saint John's University
Exercise 14.2.1:
A Lewis base must have lone pairs or non-bonding electron pairs so that it can donate them to a Lewis acid.
Alternatively, in some cases the electrons in a π-bond can be donated instead, so sometimes compounds with π-bonds can be Lewis basic.
Exercise 14.2.2:
a. not a Lewis base
b. not a Lewis base
c. Lewis base
d. Lewis base
e. Lewis base
Exercise 14.3.1:
A Lewis acid atom attracts electrons from a Lewis base.
The most common feature of a Lewis acid is an atom that is not "electronically saturated" or has not filled its octet. For example, an aluminum with only six electrons rather than eight is Lewis acidic.
Other atoms, like transition metals, have "octets" of eighteen electrons, so having fewer than eighteen electrons in their valence shell can make these atoms Lewis acidic.
Exercise 14.3.2:
a. Lewis acid
b. Lewis acid
c. not a Lewis acid
d. Lewis acid
e. not a Lewis acid
Exercise 14.3.3:
a)
b) cations
c) possibly ion-dipole forces; alternatively, the oxygen atoms could act as Lewis bases, donating lone pairs to a cation.
d)
e) anions
f)
g)
h) When the chloride ion binds to the sensor molecule it gives the overall complex a negative charge and addition of the potassium ion cancels out this charge.
Exercise 14.4.1:
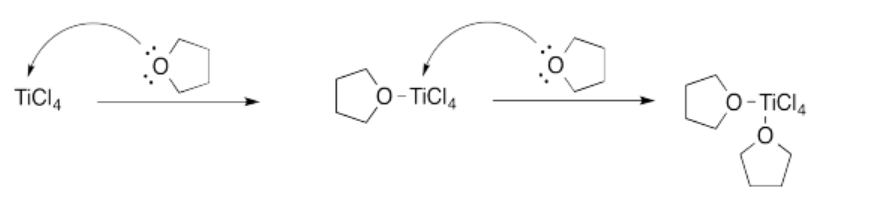
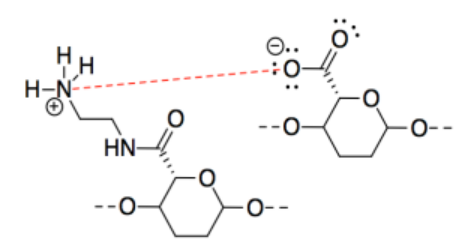
Exercise 14.4.3:
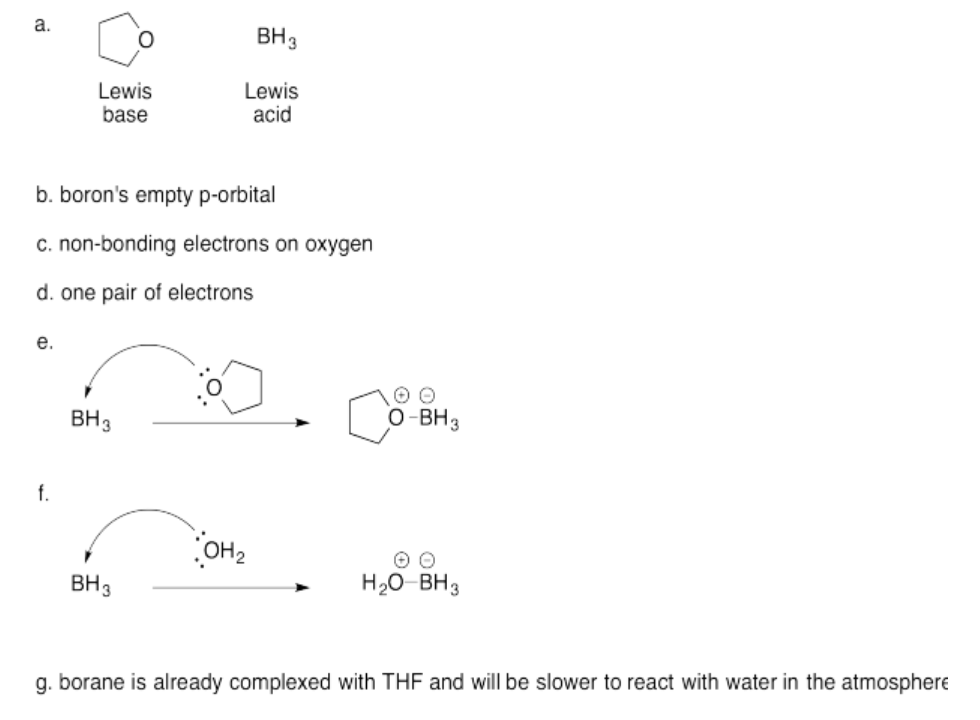
Exercise 14.4.5:
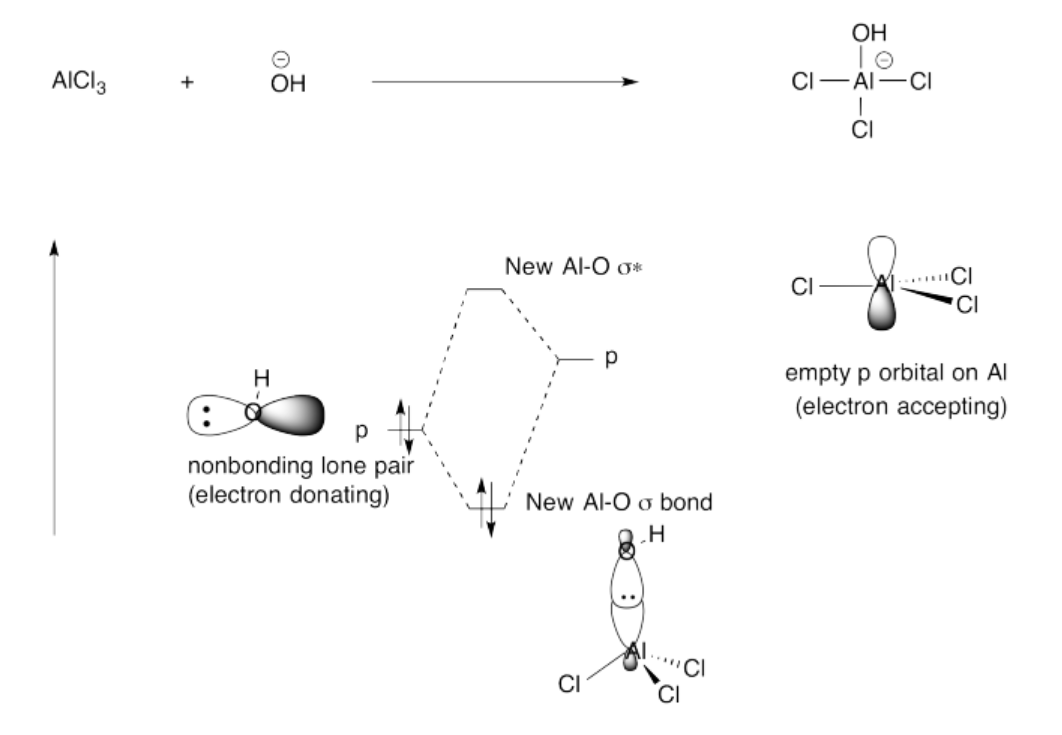
Exercise 14.4.6:
a)
b)
- The benzene rings take up more space than a fluorine atom; they may get in the way, making it harder for the water molecule to approach.
Also, the aromatic rings may be able to form a conjugated system with the empty p orbital; if that p orbital is partially filled, the boron atom becomes less Lewis acidic.
- A larger group than fluorine may cause more steric hindrance. Replacing the two fluorines closest to the boron on each arene (aromatic ring) with a CH3 or CF3 group would slow down the formation of an adduct. Alternatively, a less electronegative group than fluorine would also make the boron seem less positive; a CH3 or OCH3 are two possibilities.
- An even more electron-withdrawing group than fluorine would make the Lewis acid more reactive. Examples include nitro (NO2) and carbonyl groups (such as CH3C=0); these groups are resonance-withdrawing. Alternatively, a smaller group such a hydrogen would lower steric resistance, but would also lead to lower electrophilicity at boron, owing to the lower electronegativity of hydrogen compared to fluorine.
Exercise 14.4.7:
a) (i)
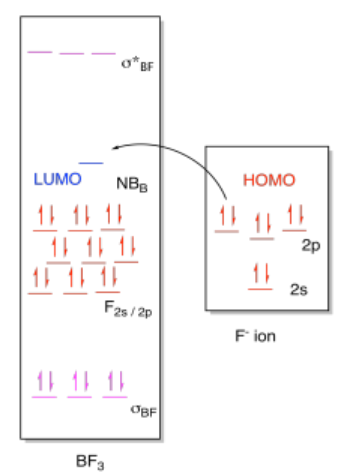
(ii)
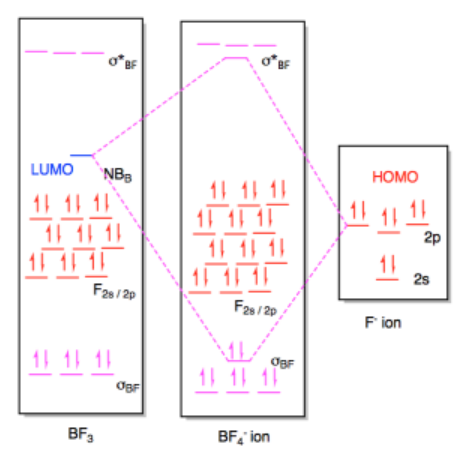
b) (i)
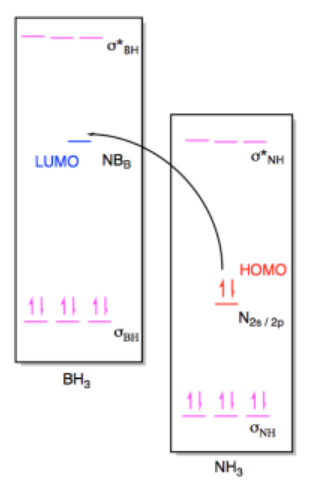
(ii)
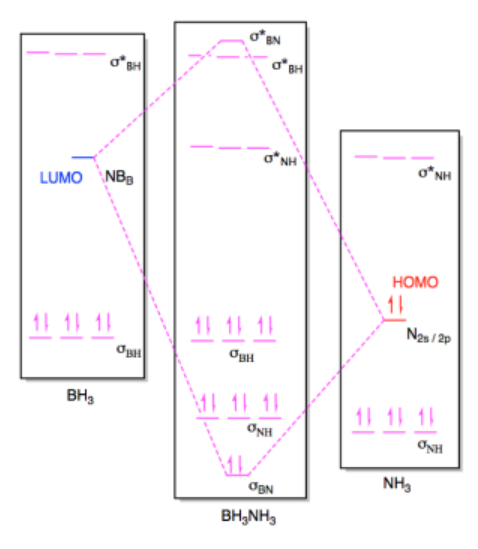
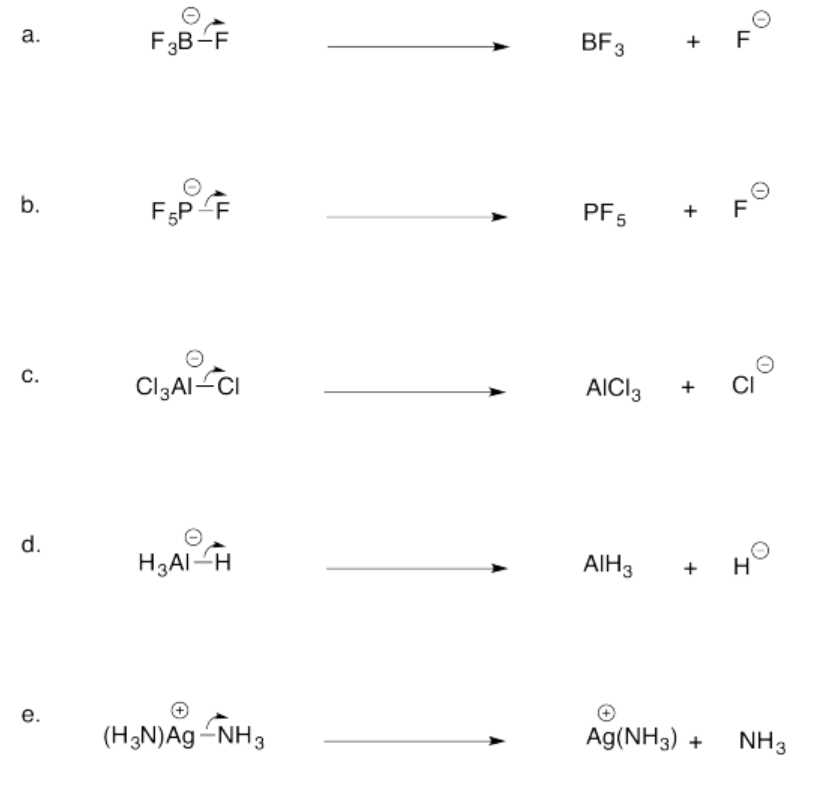
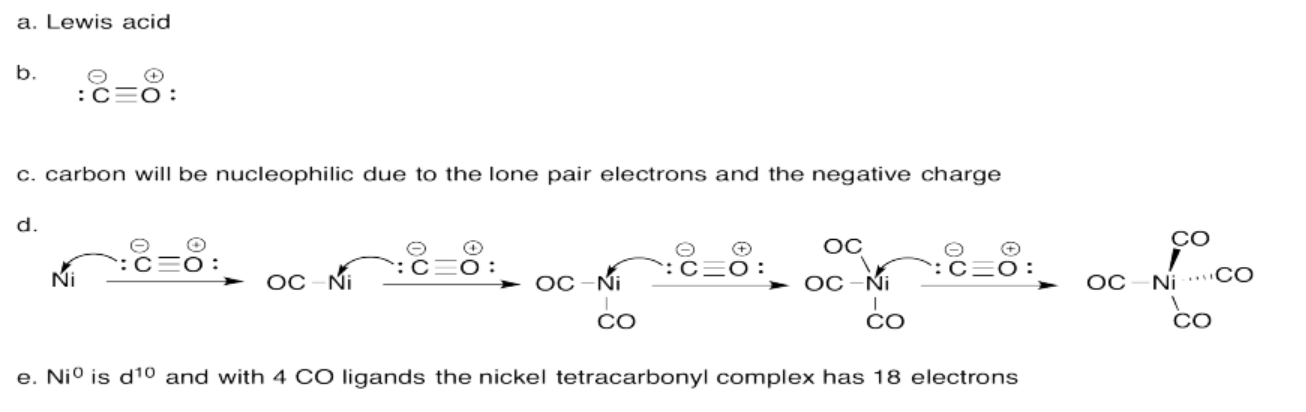
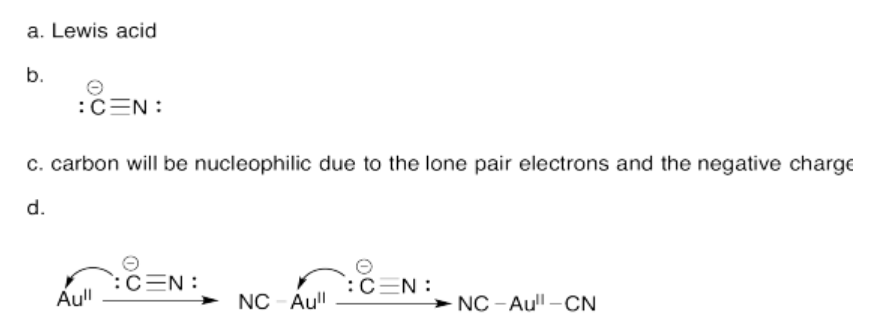

Exercise 14.7.3:
a)
b)
c)
d)
e) aromatic
f)
g)
h) This nitrogen atom can donate a lone pair of electrons without disturbing the aromatic character of the molecule.
i)
j)
k) Each side chain has a Lewis base with a lone pair of electrons to donate the copper ion.
l)
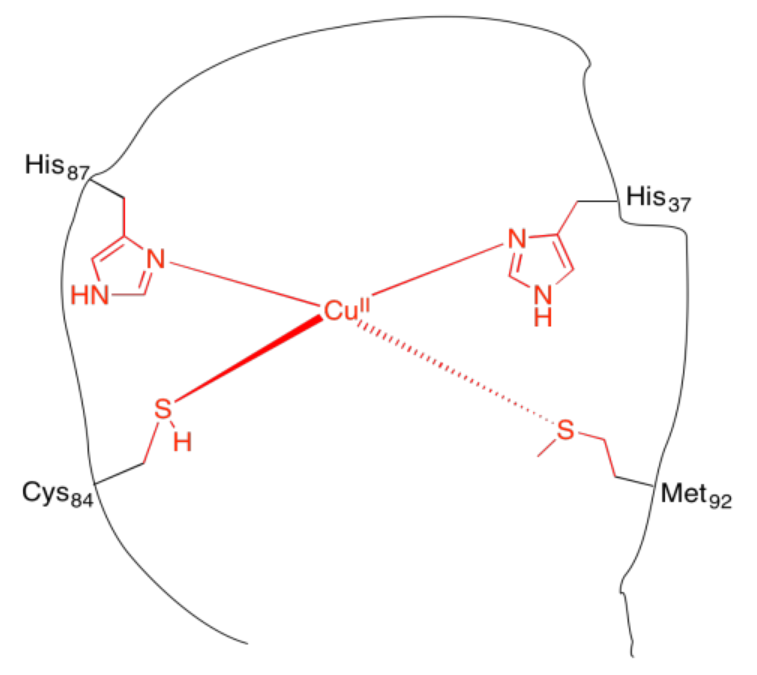
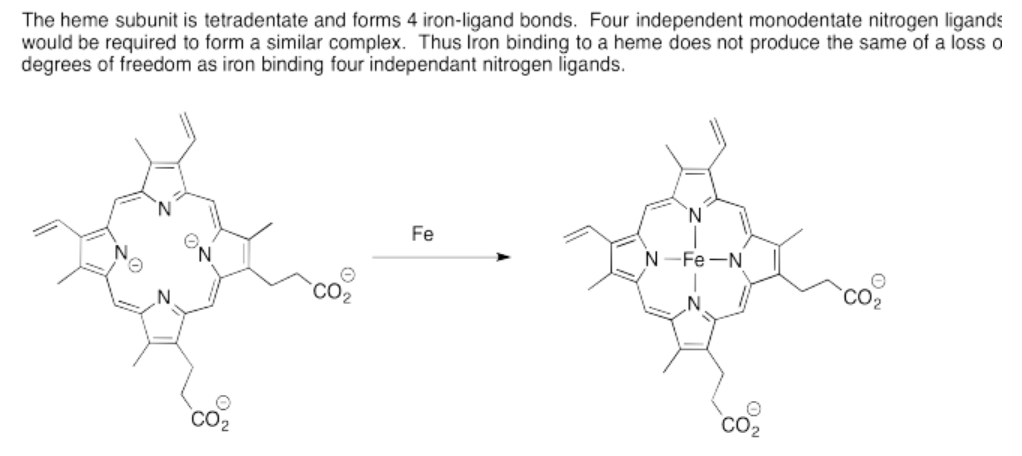
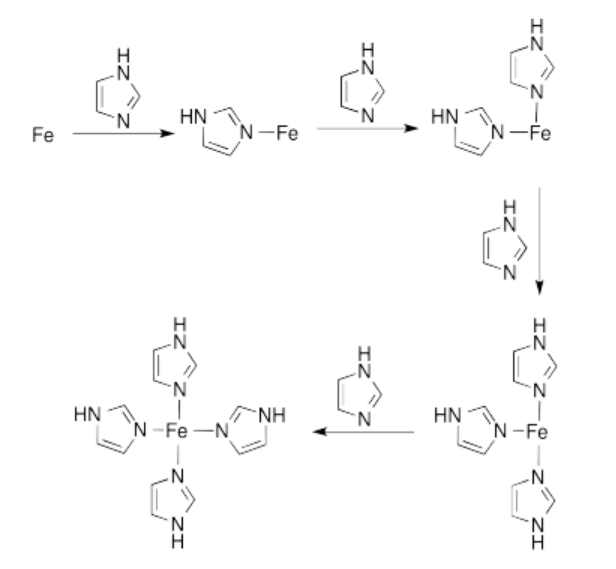
m) CN = 4
n) tetrahedral
o) Cu: 11 e-
Cu(II): 9 e-
\[4 \times 2e^{-} = 8 e^{-} \nonumber\]
\[8e^{-} + 9 e^{-} = 17 e^{-} \nonumber\]
p) If the imidazole becomes occupied by a proton, then the imidazole no longer has the lone pair to donate to the copper ion.
q) trigonal
Exercise 14.8.1:
Exercise 14.8.2:
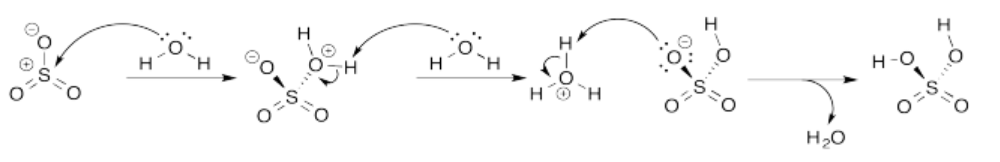
Exercise 14.8.3:
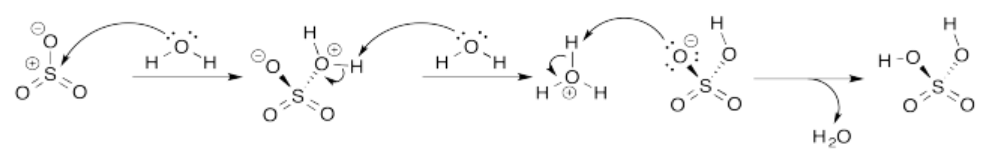
Exercise 14.8.4:
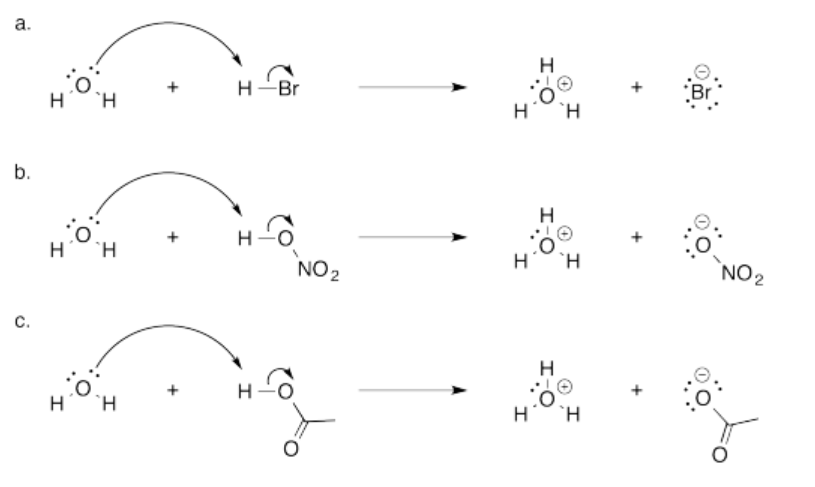
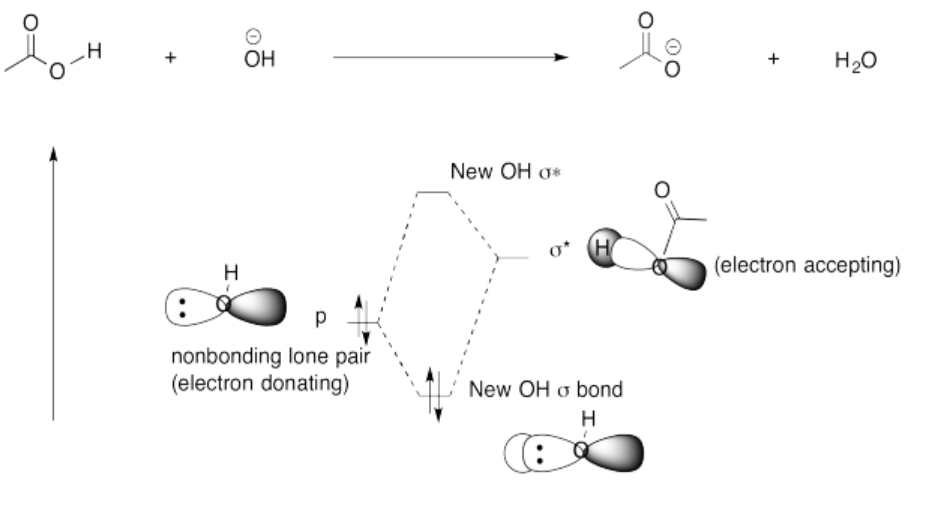
- The O-H bond is polar covalent. The oxygen is much more electronegative than the hydrogen, so the bond is easily ionized to give H+.
- In a case like NaOH, the electronegativity difference between the sodium and oxygen is much greater than the electronegativity between the oxygen and the hydrogen. The Na-O bond is ionic. The removal of a proton from hydroxide ion is harder than the removal from a proton from hydroxide. It would make an oxide anion, O2-; that buildup of negative charge is more difficult than the formation of -OH.
Exercise 14.8.5:
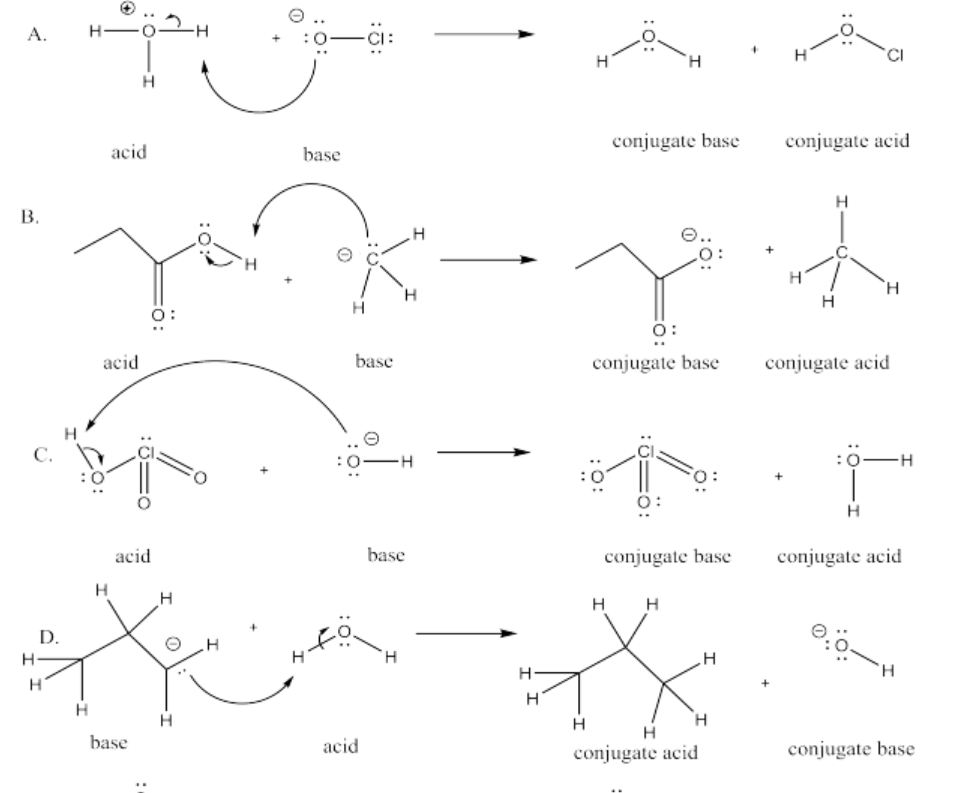
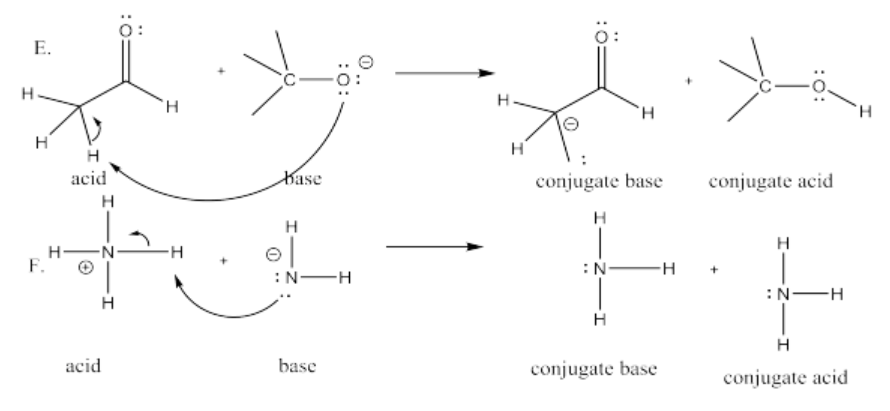
Exercise 14.8.6:
a) i)

ii)
b) i)
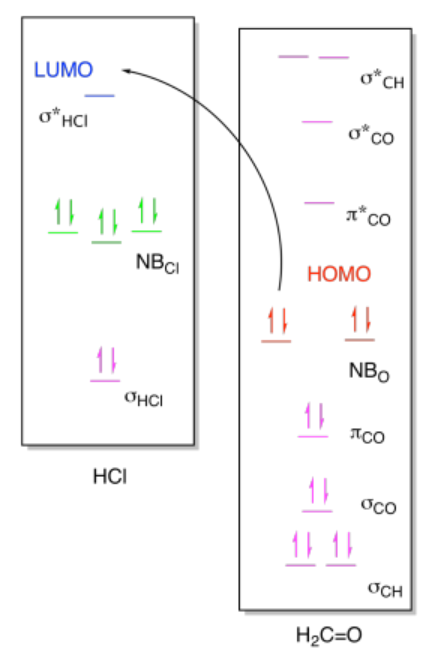
ii)
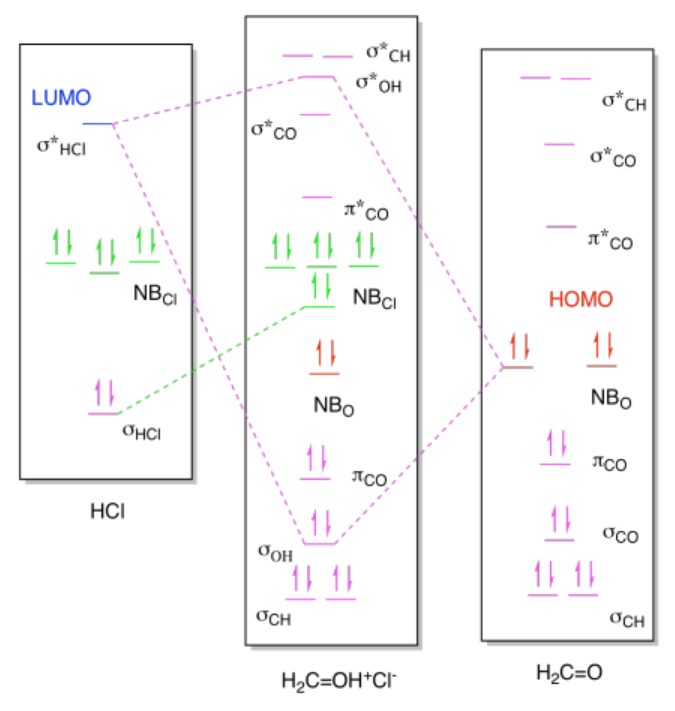
Exercise 14.9.1
a) HNO3 (pKa = -1.3); HNO2 (pKa = 3.3)
HNO3 is a stronger Brønsted acid compared to HNO2
b) H2Se (pKa = 3.9); H2O (pKa = 14)
H2Se is a stronger Brønsted acid compared to H2O
c) HCl (pKa = -8); H2SO4 (pKa = -3)
HCl is a stronger acid compared to H2SO4
d) Ba(OH)2 (pKa > 50); HSeO3 (pKa = 6.6)
HSeO3- is a stronger Brønsted acid compared to Ba(OH)2
Exercise 14.9.2:
a) NH4+ (pKa = 9.2); HN3 (pKa = 4.7)
NH3 is a stronger Brønsted base compared to N3-
b) HCN (pKa = 9.4); HSCN (pKa = 4)
-CN is a stronger Brønsted base compared to -SCN
c) NH3 (pKa = 35); H2O (pKa = 14)
-NH2 is a stronger Brønsted base compared to HO-
Exercise 14.10.1
- HClO4
- H3PO4
Exercise 14.11.1:
a) H2S or SiH4 b) GaH3 or AsH3
c) PH3 or AlH3 d) H2Se or HBr
Exercise 14.11.2:
a) +NH4 or NH3 b) -PH2 or PH3
c) H2O or +NH4 d) +H3O or CH4
Exercise 14.11.3:
a) H2S or H2Te b) GeH4 or SnH4
c) HCl or HBr d) NH3 or AsH3
Exercise 14.11.4:
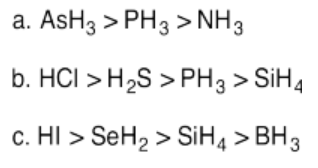
Exercise 14.11.5:
a. Polarizability would not be useful as the atom that will be anionic in the conjugate base is the same (oxygen).
b. Se is larger and therefore more polarizable leading to H2Se being more acidic compared to H2S.
c. Ge is larger and therefore more polarizable leading to GeH4 being more acidic compared to SiH4.
d. Polarizability would not be useful as the atoms are next to one another in the same row and therefore likely to have the same polarizability.
Exercise 14.11.3:

Exercise 14.12.1:
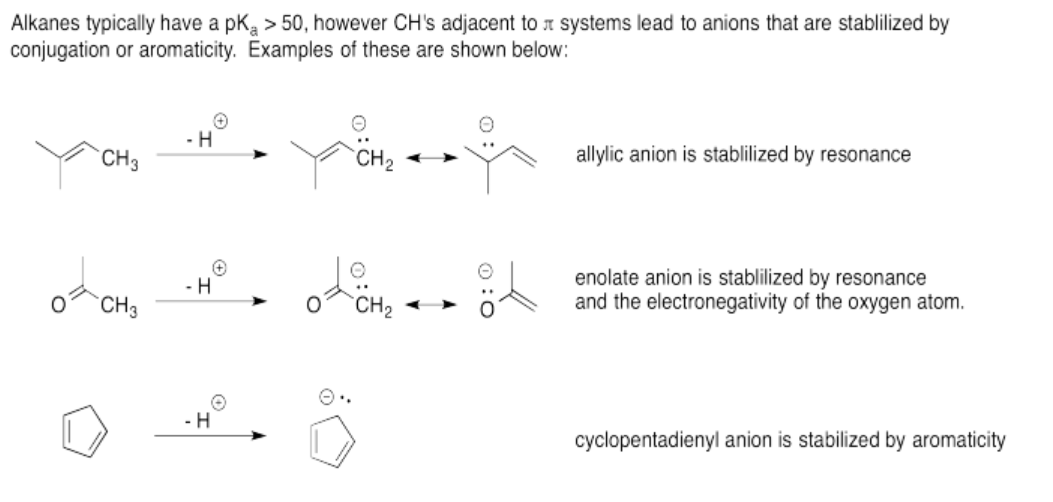
Exercise 14.12.2:
a. Vinyl alcohol would be more acidic as its anion is stabilized by resonance while the anionic charge on ethoxide would be localized on the oxygen atom.
b. Nitromethane would be more acidic as its anion is stabilized by resonance while the anionic charge of the trimethylamine anion would be localized on the carbon atom.
c. Acetonitrile would be more acidic as its anion is stabilized by resonance while the anionic charge of the trimethylamine would be localized on the carbon atom.
Exercise 14.12.3:
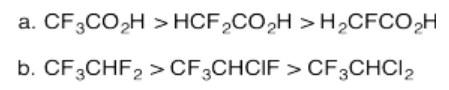
Exercise 14.12.4:
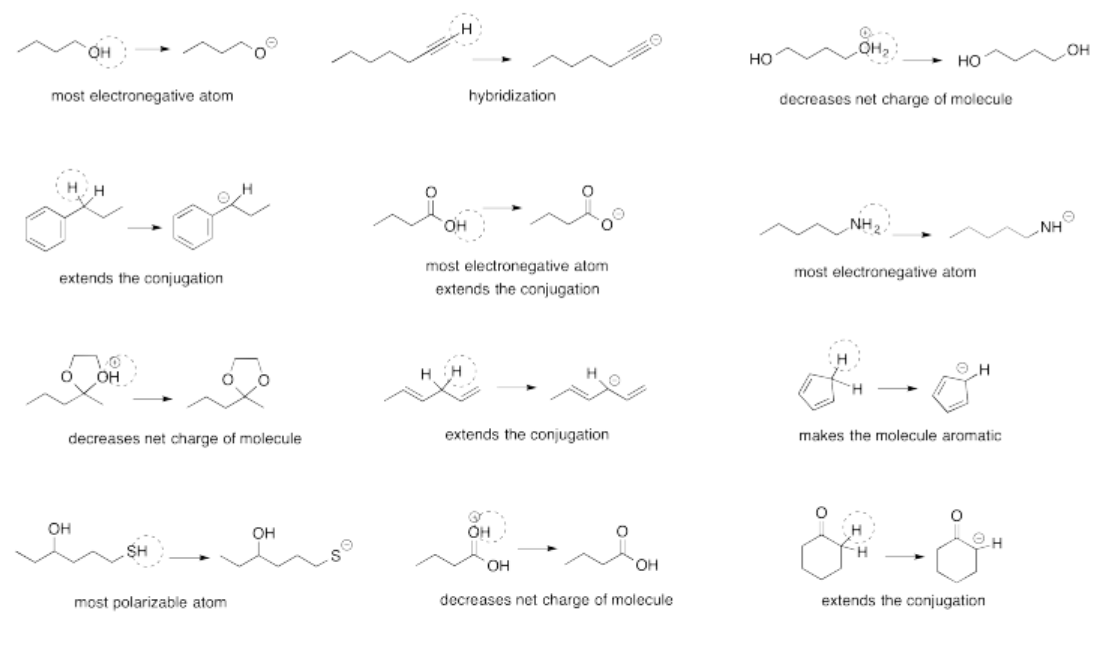
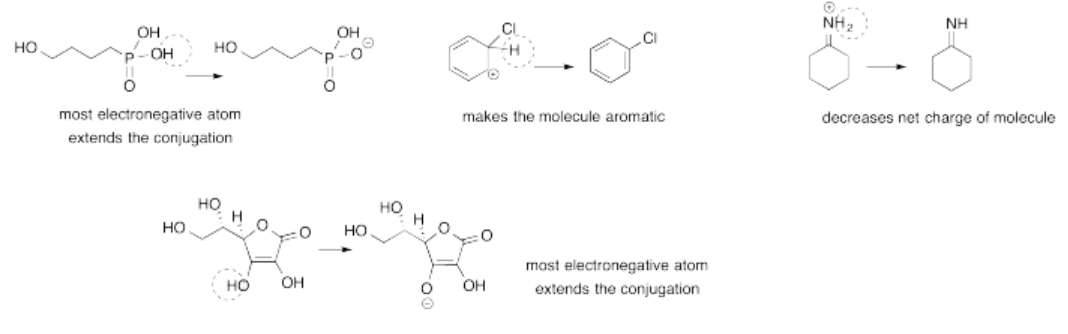
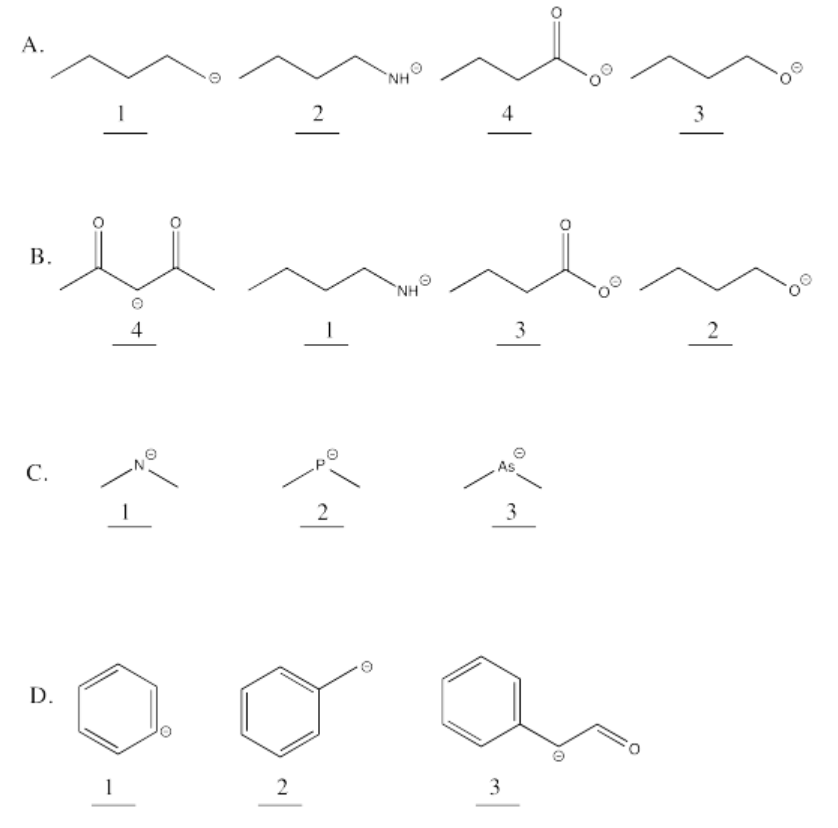
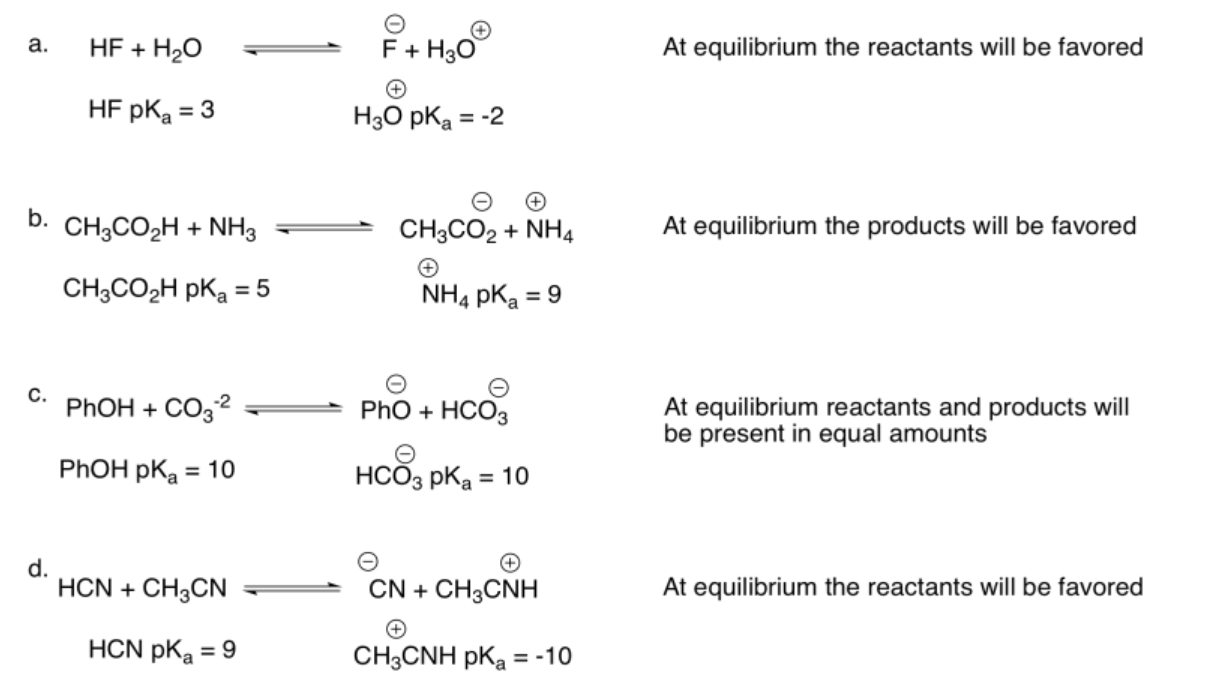
Exercise 14.14.2:
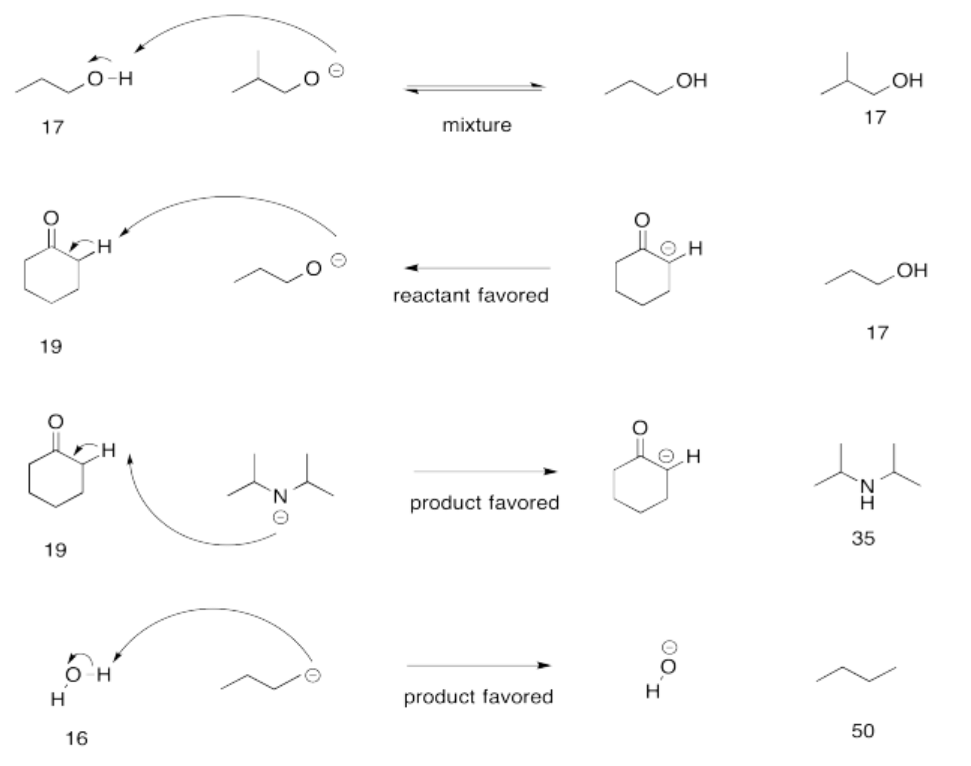
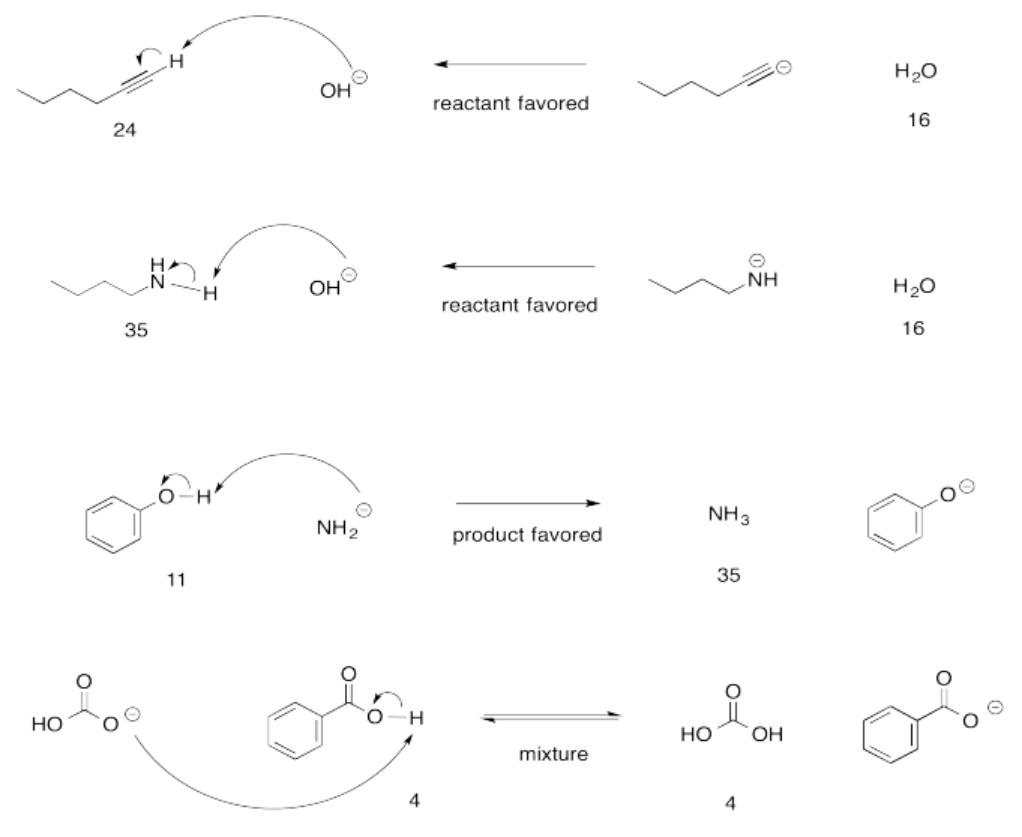
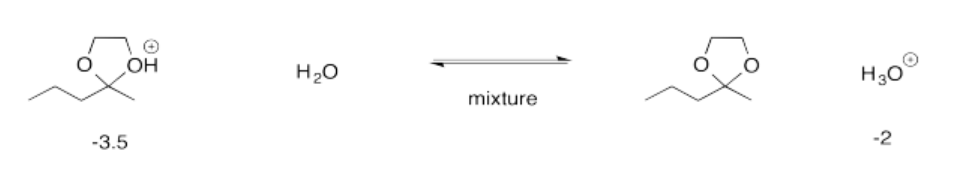
Exercise 14.15.1:
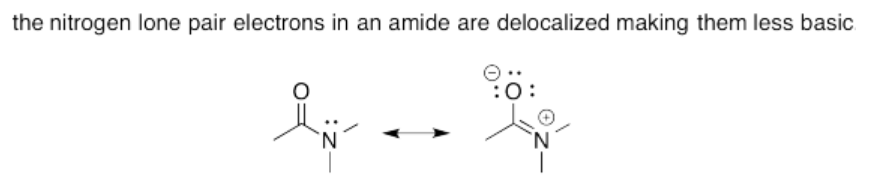
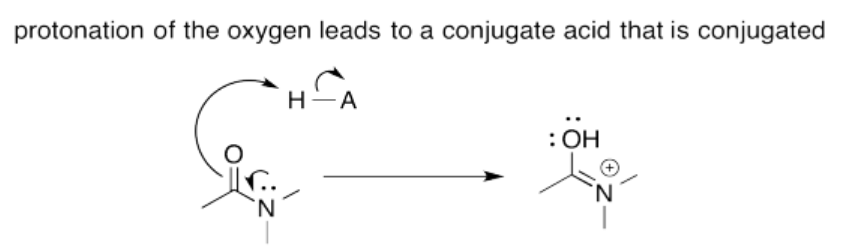
Exercise 14.15.4:
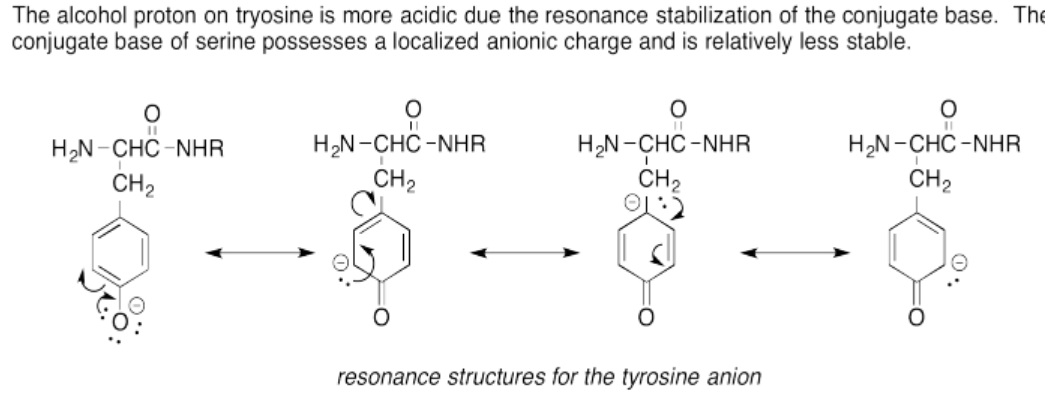
The thiol proton on cystine is more acidic due to the polarizability of the sulfur anion stabilizing the conjugate base's anionic charge. The oxygen anion is less polarizable and therefore less stable rendering the alcohol proton less acidic.
Exercise 14.15.5:
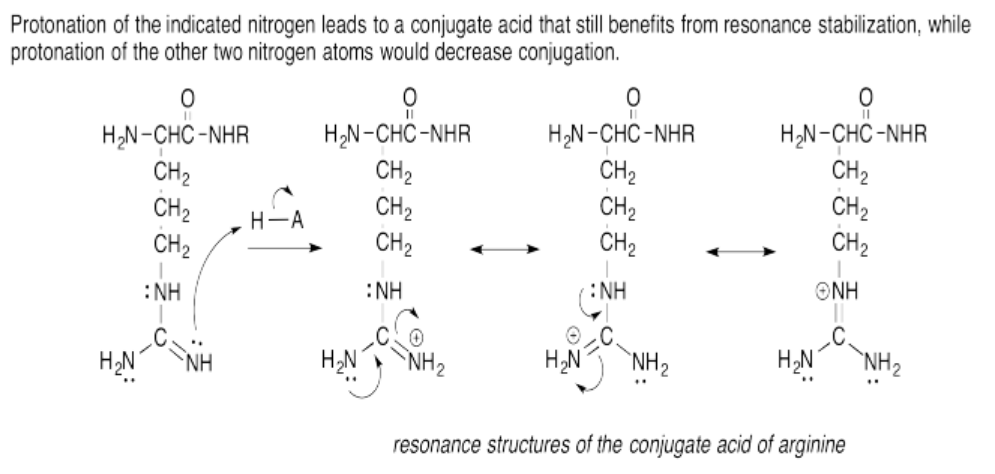
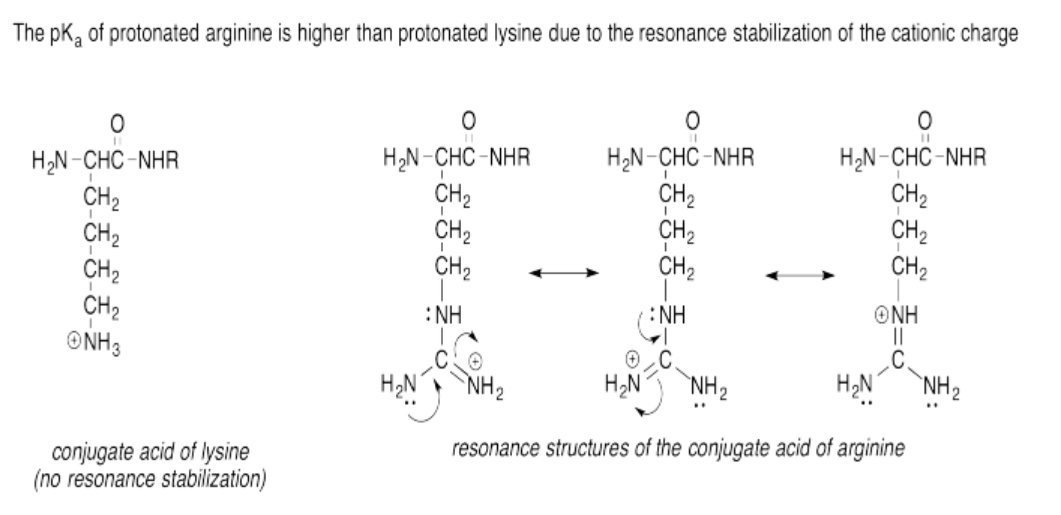
Exercise 14.15.6:
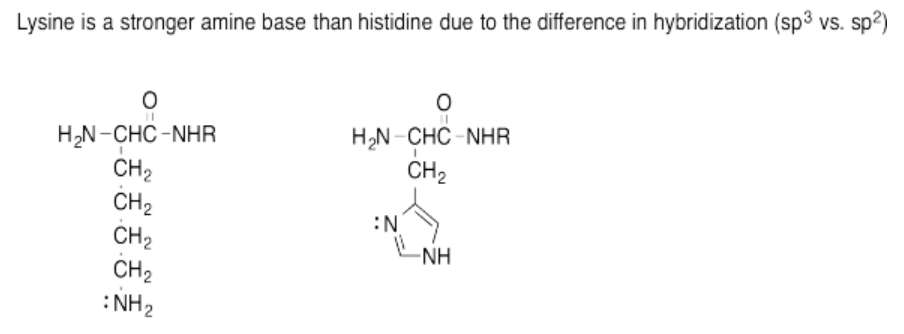
Exercise 14.15.7
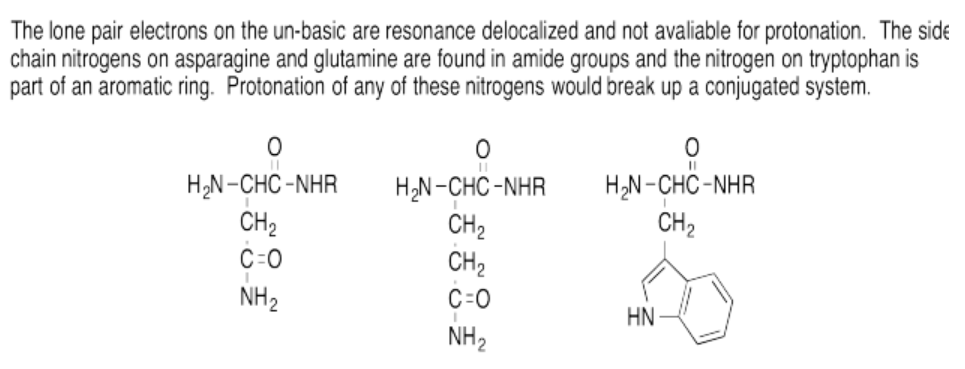
Exercise 14.16.1:
Ammonium bromide is more soluble because in water it can participate in both ion-dipole and hydrogen-bonding, while sodium bromide only benefits from ion-dipole interactions.
Exercise 14.16.2:
Hydrogen cyanide will be have a lower pKa in water as the resulting cyanide anion will be stabilized by both ion-dipole and hydrogen-bonding interactions. Hydrogen cyanide would have a higher pKa in pentanes as the resulting cyanide anion would only experience ion-induced dipole interactions which are relatively weak.
Exercise 14.17.1:
a. \(K_{a} = \frac{[NC^{-}][H_{3}O^{+}]}{[HCN]}\)
b. \(K_{a} = \frac{[HS^{-}][H_{3}O^{+}]}{[H_{2}S]}\)
c. \(K_{a} = \frac{[H_{2}N^{-}][(CH_{3})_{2}SOH^{+}]}{[HCN]}\)
Exercise 14.17.2:
a. pKa = -6
b. pKa = 9
c. pKa = 24
d. pKa = 16
Exercise 14.17.3:
a. Ka = 103.5
b. Ka = 10-4.3
c. Ka = 10-25
d. Ka = 10-9
Exercise 14.17.4:
At equilibrium formation of the Lewis acid-base complex is slightly favored.
Exercise 14.17.5:
At equilibrium formation of the Lewis acid-base complex of dimethylether and BF3 is more favored than the corresponding diethylether BF3 complex. This is likely due to the decreased steric bulk of the methy groups compared to the ethyl groups.
Exercise 14.17.6:
a. \(K_{eq}= \frac{[(NH_{3})_{2}PtCl_{2}]}{[(NH_{3})PtCl_{2}][NH_{3}]}\)
b. \(K_{eq} = \frac{[Mo(CO)_{6}]}{[Mo(CO)_{5}][CO]}\)
c. \(K_{eq}= \frac{[FeCl_{4}^{-}]}{[FeCl_{3}][Cl^{-}]\)
Exeercise 14.18.1:
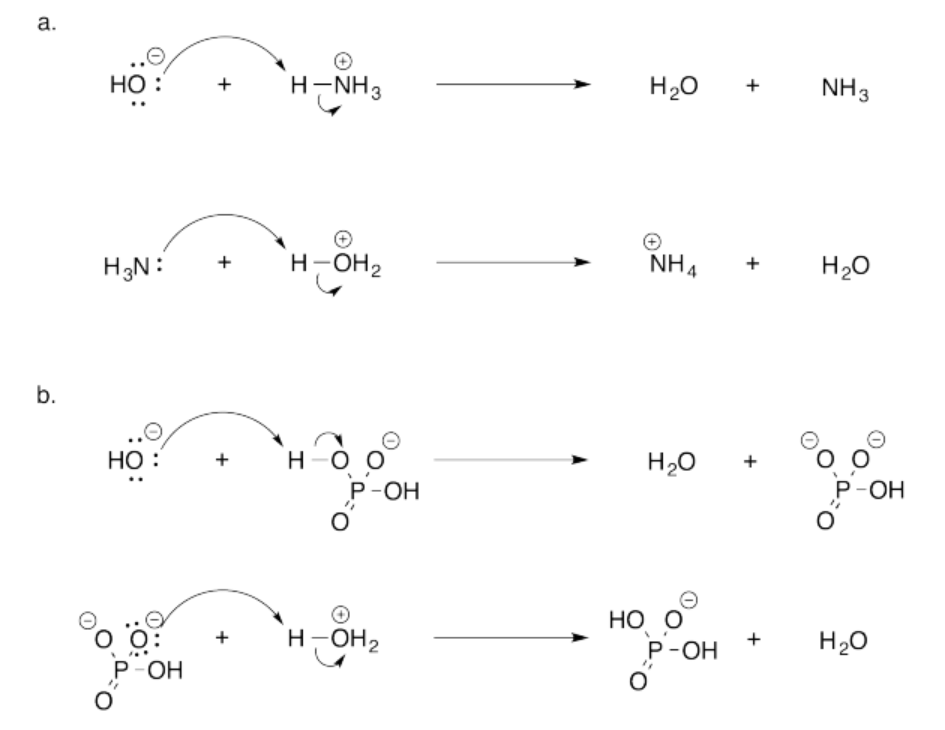
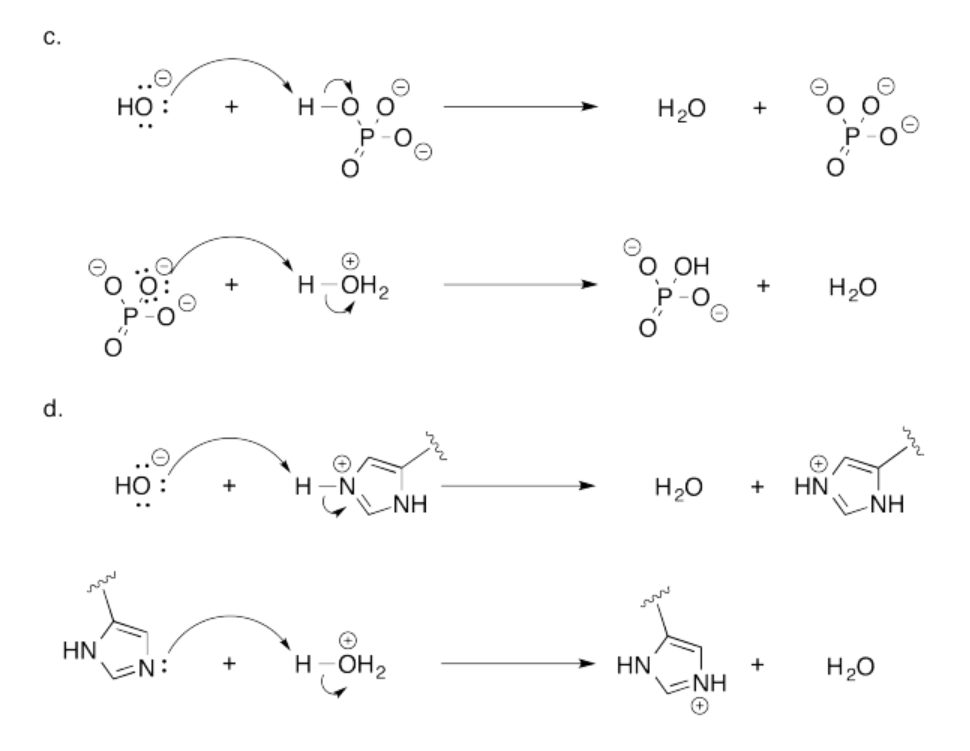
Exercise 14.19.1:
- all bases have lone pairs.
- F, N, and O have lone pairs. They can be bases.
Their order of basicity would be N > O > F.
These three elements come from the same row of the periodic table and so they are of similar sizes. However, N is the least electronegative and F is the most electronegative. N is most able to donate electrons and most able to support a positive charge, compared to the other two.
c)
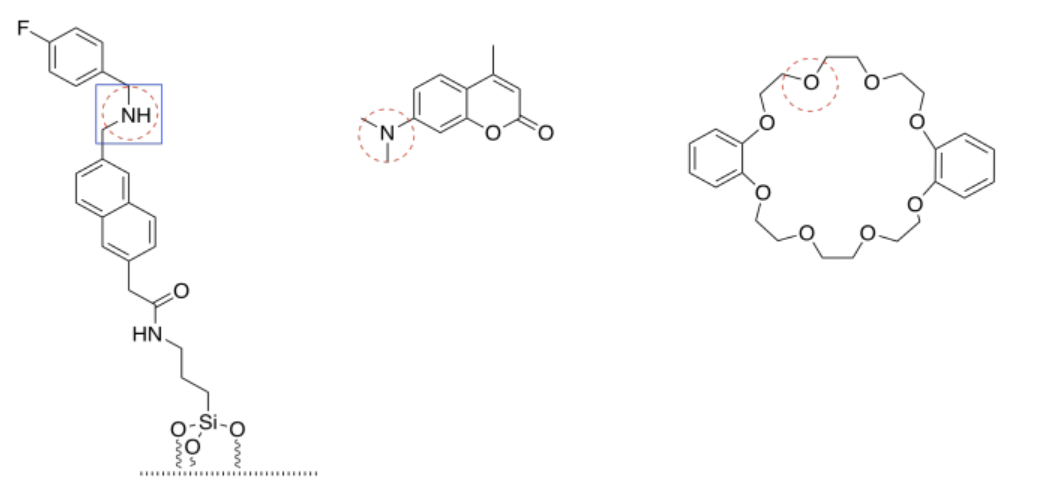
If possible, a nitrogen is circled in each molecule. The cap has only basic oxygen atoms. If there is a choice between two nitrogens (in the tether) or two oxygens (in the cap), the non-conjugated lone pair would be most basic. Conjugated lone pairs are held in place by their stable interaction with their neighbors.
The most basic of all is the amine (non-conjugated) nitrogen in the tether.
d)
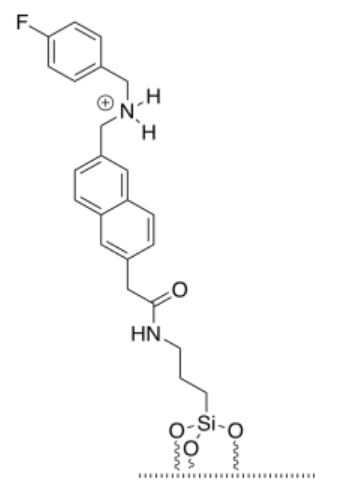
e) The cap and tether would be bound by hydrogen bonding. However, hydrogen bonding becomes much stronger if there is an ionic component. It is not a full ionic bond because the cap does not contain an anion, but its strength is between that of a normal hydrogen bond and an ionic bond.
f) Steric crowding traps the dye between the cap above and the silica surface below. The dye is too big to squeeze past the cap.
g) The dye has been released, and is now in the water.
h) There is an equilibrium between the protonated tether and the protonated triethylamine.
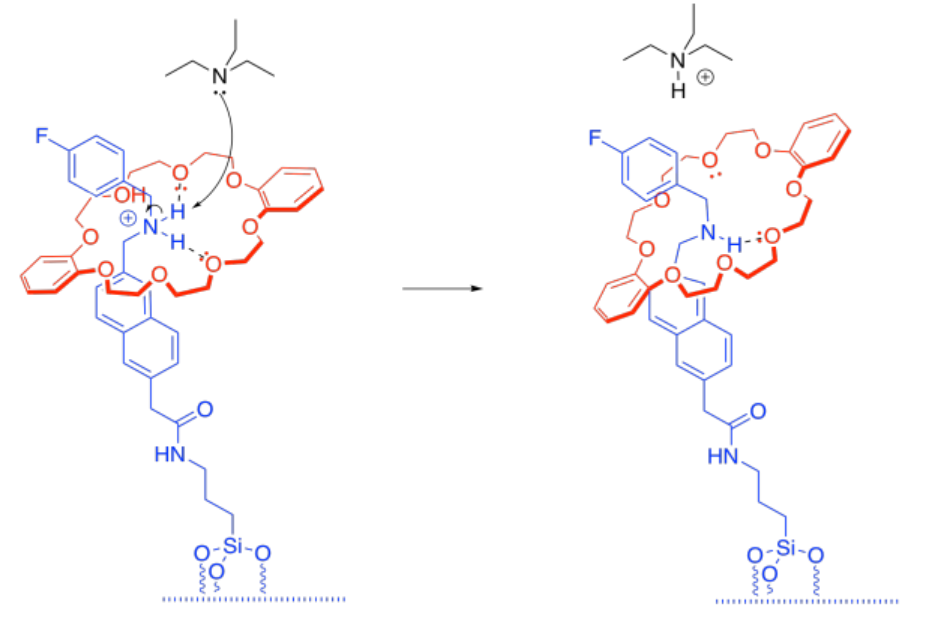
i) Once the tether has been deprotonated, the tether-cap interaction is just a normal hydrogen bond. It's still strong, but not as strong as the ion-boosted hydrogen bonding that we had before.
j) N,N-Diisopropylethylamine or Hunig's base:
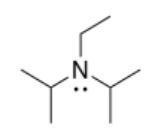
k) The lone pair in Hunig's base is much more crowded than the one in triethylamine, so it cannot remove the proton as quickly.
Exercise 14.19.2:
a)
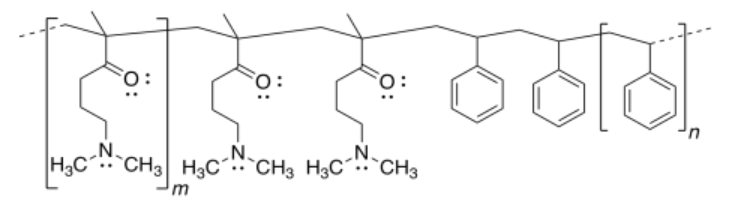
b)
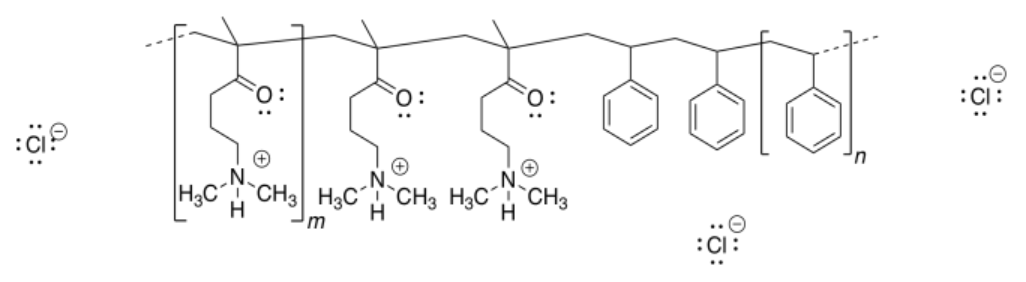
c)
d) Repulsion between the positively-charged quaternary ammonium groups (poly-CH2-N(H)(CH3)2+) would cause the nanoparticle to uncoil.
e)
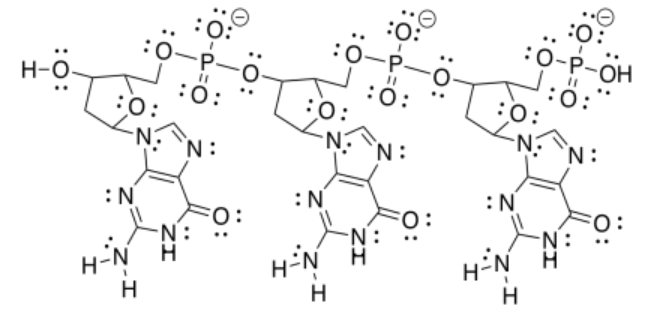
f)
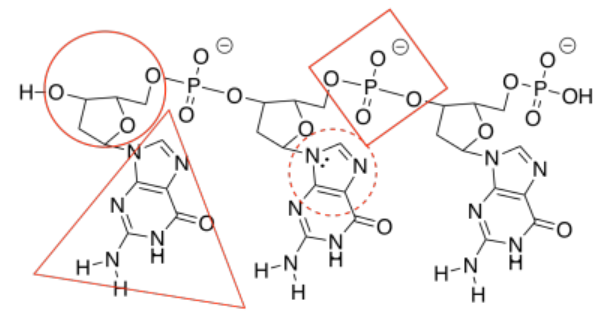
g)
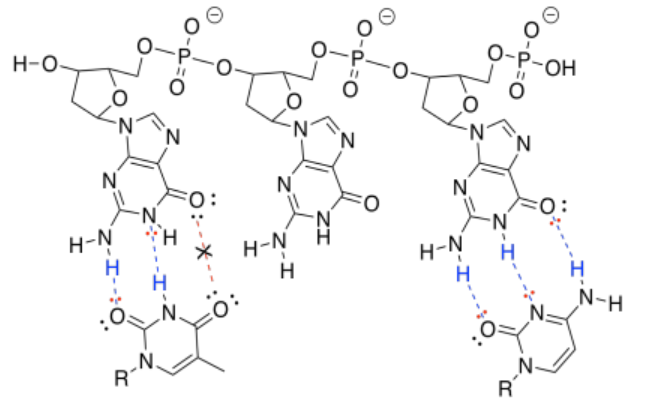
h) The one on the right forms three hydrogen bonds with the DNA and binds more tightly than the one on the left, which forms only two hydrogen bonds with the DNA.
i) The polymer is positively charged after acid treatment. It binds the negatively charged DNA via ion-ion interactions.
j) If there are enough of them, the chloride anions could "wash out" the anionic DNA. These individual anions would replace the DNA anion previously bound to the nanoparticle.
k) The triethylamine could remove the proton from the polymer chain. If the polymer chain were no longer charged, it would no longer bind the anionic DNA.
l) Also, because the nanoparticles would no longer be charged, there would no longer be repulsive forces causing the polymer to uncoil.
m)
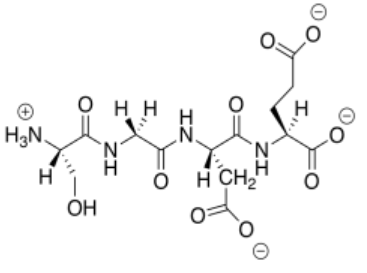
n) This anionic (overall) peptide would bind to the cationic nanoparticles, replacing the DNA.
o)
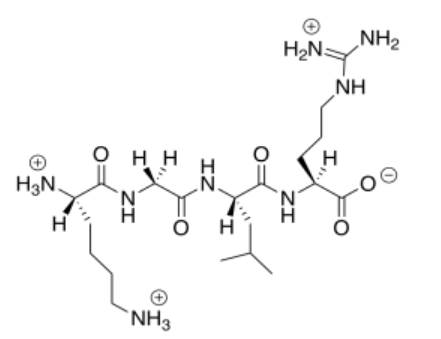
This cationic (overall) peptide would not bind to the cationic nanoparticles, so it would not displace the DNA.
Exercise 14.19.4:
a)
b)
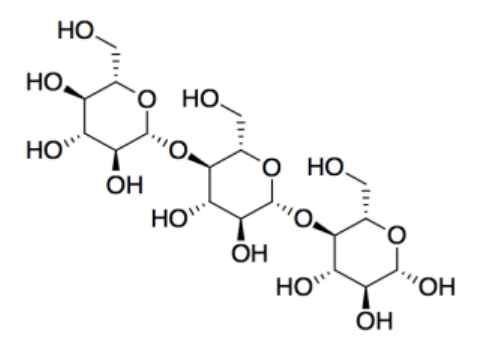
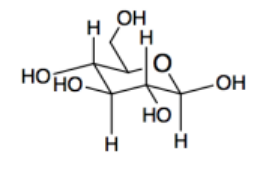
c) carbohydrates
d) Cellulose is the major component of cotton (textiles and money) and paper.
e)

f) ester
g)
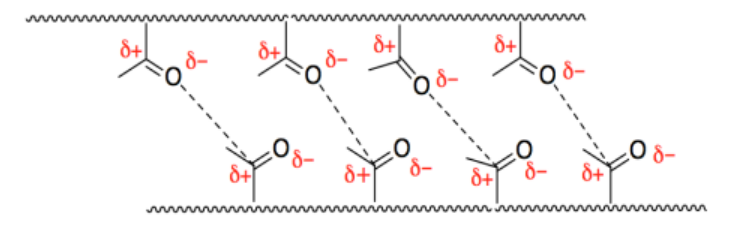
h) Body temperature is closer to 40%deg;C, so the hip would be soft and rubbery. That would making walking a little unpredictable.
i)
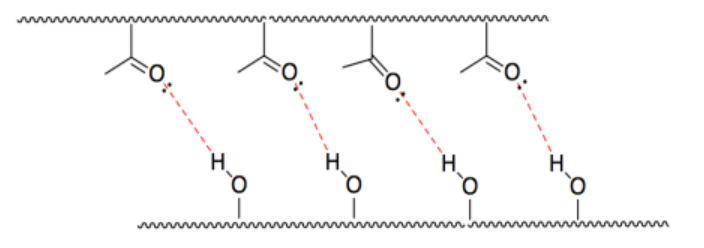
j) HCl is acidic because the H-Cl bond is polarized with electrons closer to Cl.
k) NaOH is basic because the Na-O bond is more polarized, with electrons closer to O.
l) CNC-CO2H is most acidic because of the resonance stabilized anion in the conjugate base.
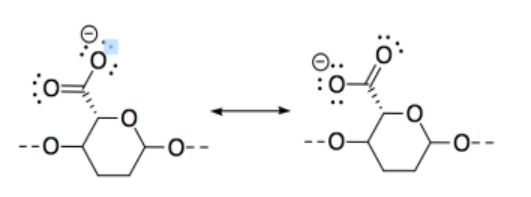
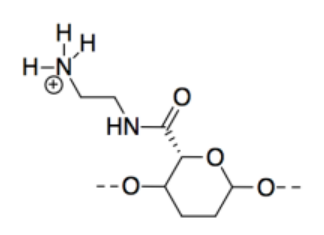
m) CNC-NH2 is most basic because, although all three compounds possess lone pairs, the lone pair on the nitrogen is on a less electronegative atom than the lone pairs on oxygens, so it is more readily donated to a proton.
n) This one may seem counterintuitive. The intermolecular forces at pH 11 are ionic, which should be stronger than hydrogen bonding. However, interactions between two anionic chains are repulsive, which would decrease the attraction between neighbouring CNC chains.
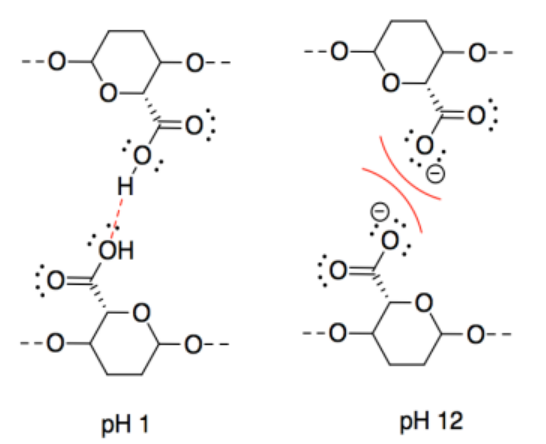
o) The intermolecular forces at pH 3 are ionic, which should be stronger than hydrogen bonding. However, interactions between two cationic chains are repulsive, which would decrease the attraction between neighbouring CNC chains.
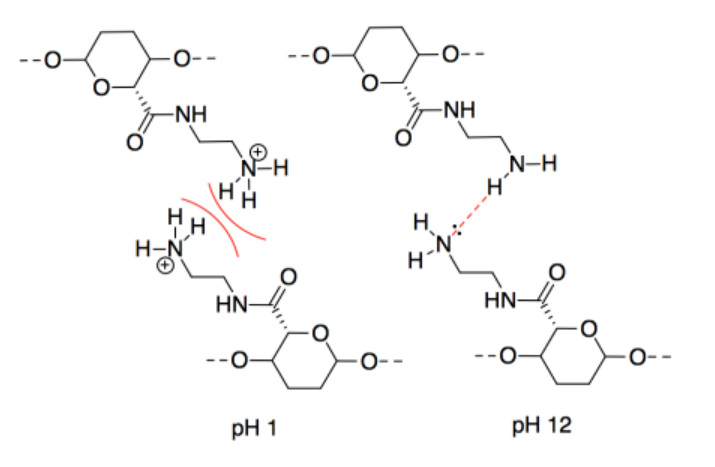
p) A proton would be transferred from the acidic site to the basic site. The oppositely charged CNC chains would attract each other strongly.