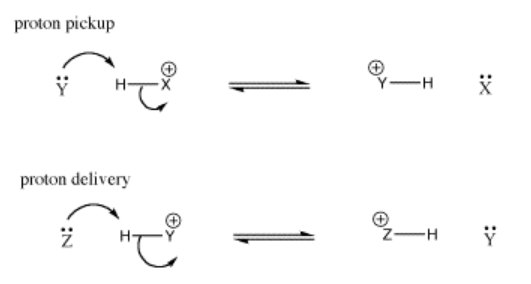
14.8: Proton Transfer from One Basic Site to Another and Molecular Orbital Interactions in Proton Transfers
- Page ID
- 193777
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)Acidity involving protons is complicated because of the exchange of one base for another. The proton has attracted the electron pair from the base via its partial positive charge. Once the base arrives, however, the proton cannot bind to the new base and still retain its tie to the base to which it was already bound. A hydrogen can only have 2 electrons in its valence shell, not four.
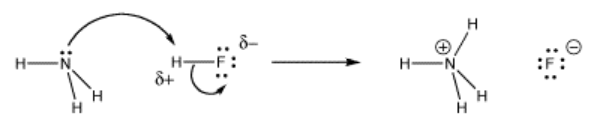
What begins very much like the formation of an intermolecular hydrogen bond goes one step further. The proton forms a full bond to the incoming donor, and releases its bond to its former partner.
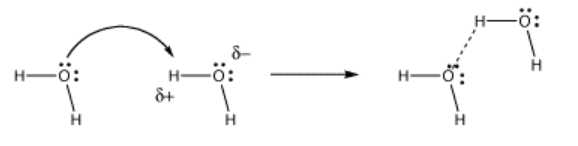
In hydrogen bonding, the lone pair is partially shared with a proton, without actually displacing the other partner from the proton. A true bond is not quite formed between the donor and the proton. However, the distinction between hydrogen bonding and proton transfer can be blurred. Often, both cases happen at the same time in a given system.
- Hydrogen atoms can normally have only one bond.
A conjugate base is any Lewis base that forms as a result of a proton transfer. This label is only relative. Any Lewis base could be called a conjugate base if, in the present circumstances, it has lost a proton. Also, a conjugate acid is any compound that has just gained a proton.
However, the terms conjugate base and conjugate acid imply that these compounds can potentially act as bases and acids, respectively. because the conjugate base has a lone pair and is a Lewis base, it could in fact take a proton again. Because a conjugate acid has a proton, it could conceivably give the proton up again. Whether or not this event will actually occur is another matter. Later, we will develop more of an understanding of when proton transfers will actually occur.
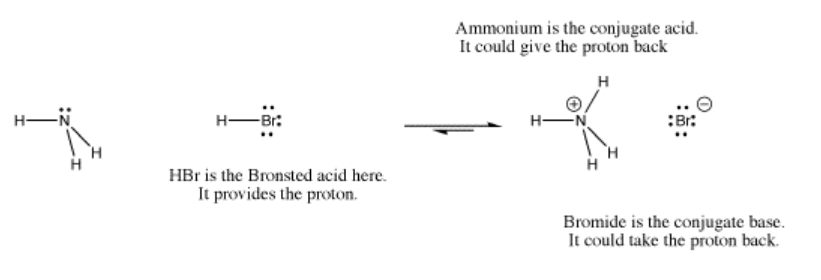
- The pair of electrons connecting the proton to the old Lewis base leave with the base. This base is called the "conjugate base".
- The proton is now bound only to the new Lewis base. This new compound is called the "conjugate acid".
We also need a new term to call the compound that provides the proton in these proton transfer reactions. The proton is the Lewis acid in these cases, but what do we call the entire compound that contains the acidic proton? This compound is commonly called a Brønsted acid. This term comes from studies of acids by Brønsted and Lowry, who independently observed that many reactions in chemistry involve proton transfers.
- A Brønsted acid is a compound that provides a proton for a base to take.
Again, the conjugate acid that forms after proton transfer is often a Brønsted acid; it could transfer the proton to another base. It could transfer the proton back to the conjugate base, reforming the starting materials. Alternatively, it could transfer the proton to a new base. In that case, the Lewis base in the first step has picked up a proton, turned into a Brønsted acid, delivered the proton to another base, and become a Lewis base again. It is like a taxicab that has picked up a passenger and dropped it off again. Sometimes in this situation, the Lewis base is called a proton shuttle.
Delivery of protons is an essential task in a wide array of chemical processes. In biological systems, histidine residues in proteins commonly function as proton shuttles (although they play many other roles as well). For example, the interconversion of aldose and ketose sugars is a basic step in a variety of biosynthetic pathways. In an aldose, a C=O group is found at the end of a carbon chain. In a ketose, this C=O group is not at the end of the chain.
An aldose can be converted into a ketose essentially by moving some protons.
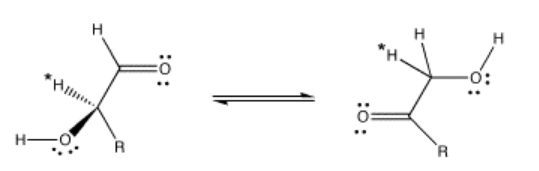
When this transformation is carried out by the enzyme, glyoxalase, studies have shown that the proton marked with an asterisk in the aldose and ketose above are exactly the same proton, having moved from one position to another. The proton has simply been moved by a proton shuttle.
The evidence for the specific proton from the aldose ending up in the ketose comes from labeling studies. In a labeling study, a different kind of proton is placed in that specific position in the aldose. This task would involve a less-common isotope such as tritium or deuterium instead of the usual protium. Deuterium and tritium can both be distinguished from protium; for example, deuterium and protium both absorb radio waves in a technique similar to medical MRI, and by using this technique it is easy to distinguish one from the other. As a result, we can tell where a deuterium is found in a particular molecule. When the aldose is then exposed to glyoxalase, we can see that deuterium show up in a specific spot in the ketose.
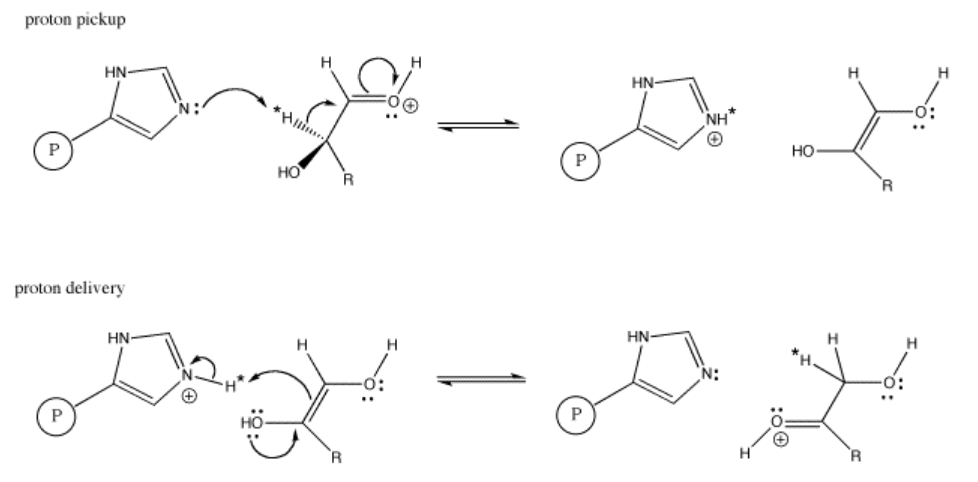
The scheme above shows only the transfer of the marked proton from one carbon to another. In addition, a proton must be placed on the oxygen of the C=O group in the aldose, and another proton must be removed from the OH group on the next carbon in the chain. Additional proton transfer steps would accomplish these tasks.
Exercise \(\PageIndex{1}\)
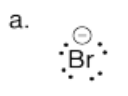
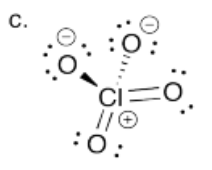
Provide the conjugate bases of the following mineral acids. In each case, assume only one proton is lost.
a) HBr b) H3PO4, connected (HO)3PO c) HClO4, connected HOClO3.
- Answer a
-
- Answer b
-
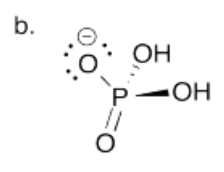
- Answer c
-
Exercise \(\PageIndex{2}\)
Provide the conjugate acids of the following bases.
a) H2O b) NH3 c) CH3NH2 d) CH3O- e) H-
Exercise \(\PageIndex{3}\)
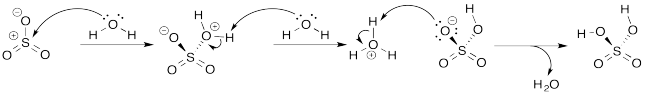
Some of the more common mineral acids that you may be familiar with from high school chemistry are formed as a result of Lewis acid-base complexation, followed by proton transfer. Use arrow formalisms to show the elementary steps that occur when the following compounds are dissolved in water.
a) sulfur trioxide, SO3, to give sulfuric acid, H2SO4 (connected (HO)2SO2).
b) nitric oxide ion, NO2+, to give nitric acid, HNO3 (connected HONO2).
- Answer a
-
Exercise \(\PageIndex{4}\)
Note that a number of common acids contain OH groups.
a) What is it about this group makes these compounds acidic?
b) Some compounds, such as NaOH or KOH, are not very acidic. In fact, they are termed Arhennius bases, meaning they are ionic compounds that dissociate in water to give hydroxide ions (OH-), rather than protons. Explain why these compounds behave differently from the acids containing the same group, and why the hydroxide ion is basic.
- Answer a
-
The O-H bond is polar covalent. The oxygen is much more electronegative than the hydrogen, so the bond is easily ionized to give H+.
- Answer b
-
In a case like NaOH, the electronegativity difference between the sodium and oxygen is much greater than the electronegativity between the oxygen and the hydrogen. The Na-O bond is ionic. The removal of a proton from hydroxide ion is harder than the removal from a proton from hydroxide. It would make an oxide anion, O2-; that buildup of negative charge is more difficult than the formation of -OH.
Exercise \(\PageIndex{5}\)
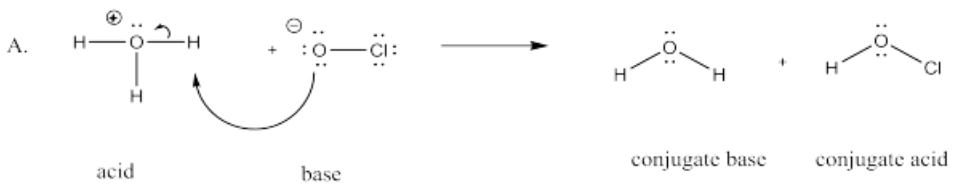
The following pairs of species will react with each other via an acid-base reaction. For each pair, complete the reaction by adding the products of the reaction, drawing a Lewis dot structure for all four reactants and products, labeling each species in the reaction as an acid, conjugate acid, base or conjugate base; and drawing arrows to show the electron flow.
A. H3O+ + OCl-
B. CH3CH2COOH + CH3-
C. HClO3 + OH-
D. CH3CH2CH2- + H2O
E. CH3CHO + (CH3)3CO-
F. NH4+ + NH2-
- Answer A.
-
- Answer B.
-
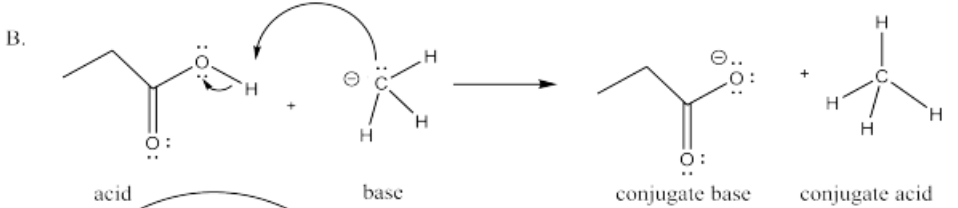
- Answer C.
-
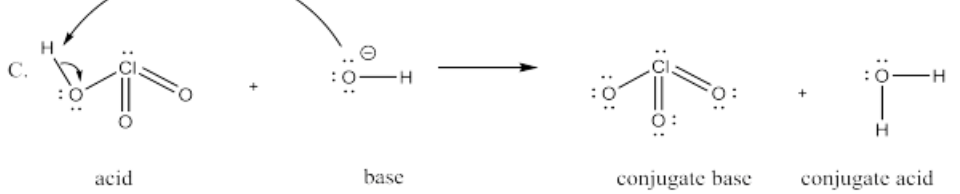
- Answer D.
-
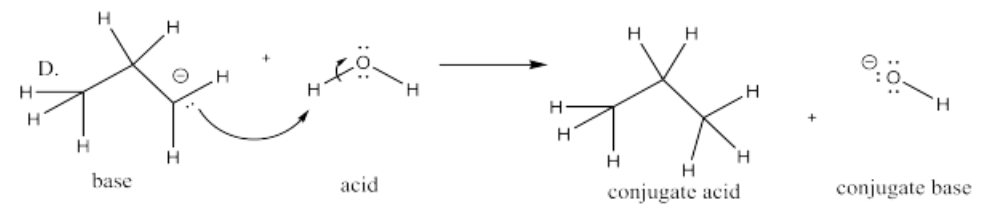
- Answer E.
-
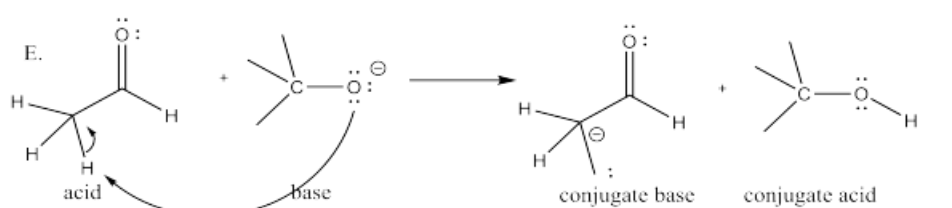
- Answer F.
-
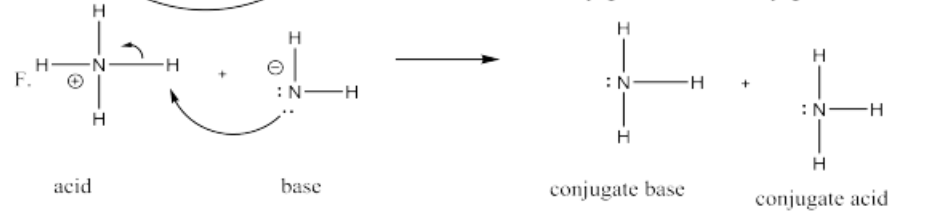
In Brønsted acidity, the Lewis acid is always a proton. However, protons aren't generally found by themselves. That's because they are such good Lewis acids; they are usually found sticking to a Lewis base already.
Earlier, we looked at how frontier orbitals are sometimes used to think about reactions. We think about the highest occupied molecular orbital on one reactant as the source of electrons. We imagine the lowest unoccupied orbital on the other reactant as the destination for the electrons.
Suppose a proton is transferred from a hydrogen chloride molecule, HCl, to a water molecule, H2O. That's what will happen if hydrogen chloride, a gas, is bubbled into some water, forming aqueous hydrochloric acid.
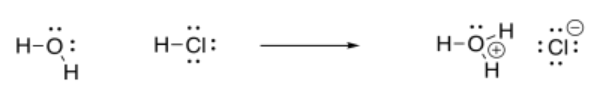
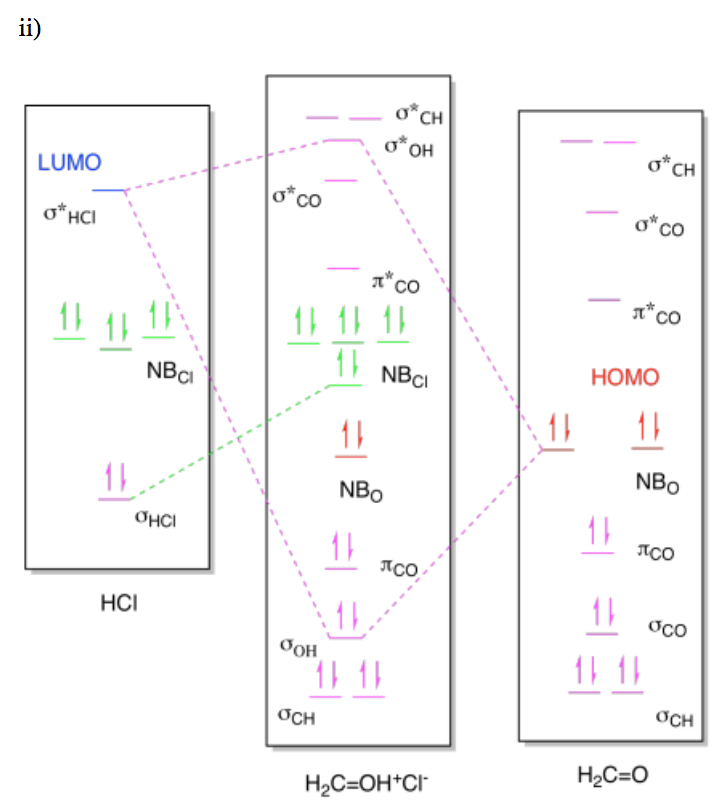
The electrons are donated from a non-bonding pair of electrons on the oxygen atom in the water molecule. That part seems pretty straightforward from the Lewis structure, and we see the same idea conveyed in the MO diagram. But where do these electrons go? There is no obvious acceptor in the Lewis structure, because the hydrogen atom has two electrons and the chlorine has eight, so both octets are satisfied. We need to bring in the idea of electronegativity to see that the two electrons in the H-Cl bond are not shared evenly; there is a parital negative charge on chlorine and a partial positive charge on hydrogen. That fact make hydrogen seem like the electron acceptor. Sure enough, an O-H bond has formed in the product, suggesting the oxygen atom has donated a pair of its electrons to the hydrogen.
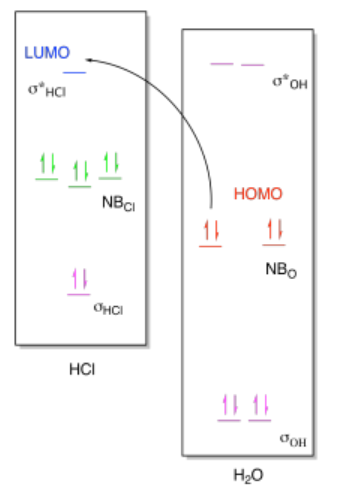
If we look at the molecular orbital picture, we see that the LUMO on HCl is an antibonding orbital, the σ*HCl. The non-bonding electrons from oxygen will be donated into the empty antibonding orbital on HCl. Remember, putting electrons in an antibonding orbital always weakens or breaks the bond. That's because the energetic advantage of dropping electrons into a low-energy bonding orbital is negated by the energetic disadvantage of raising electrons into a high-energy antibonding orbital.
As a result, there is more going on in the molecular orbital interaction diagram than just bond formation. As in previous cases, the MO diagram for the product looks a little like the MO diagrams of the two reactants added together, but there are some changes. Once again, the HOMO from the base and the LUMO from the acid mix together to form a new low-energy bonding level and a new high-energy antibonding level in the product. Because the LUMO is an antibonding orbital, however, there is an additional consequence: the corresponding bondng orbital in the acid becomes a non-bonding orbital, because that bond must be broken.
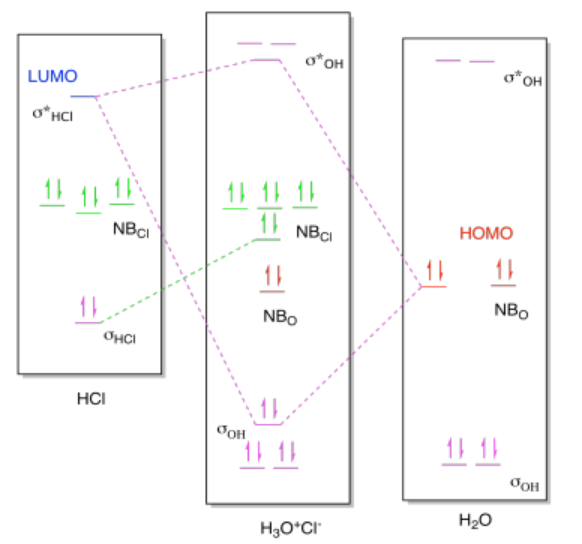
In this case, the MO picture reminds us of something in addition to the bond formation, and that i the bond-breaking that accompanies proton transfers.
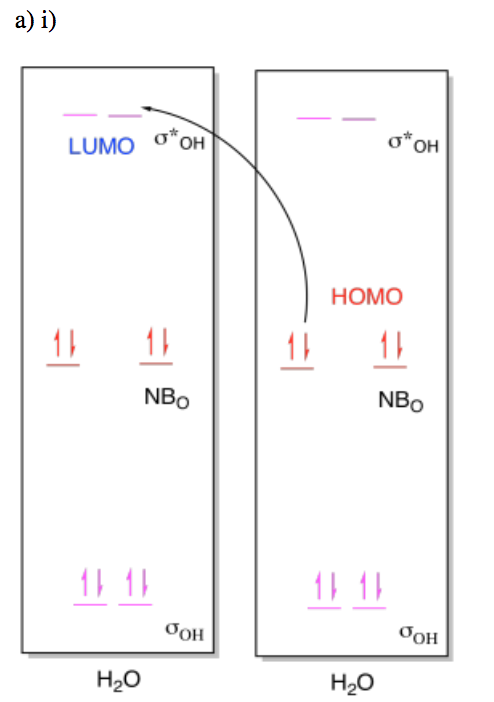
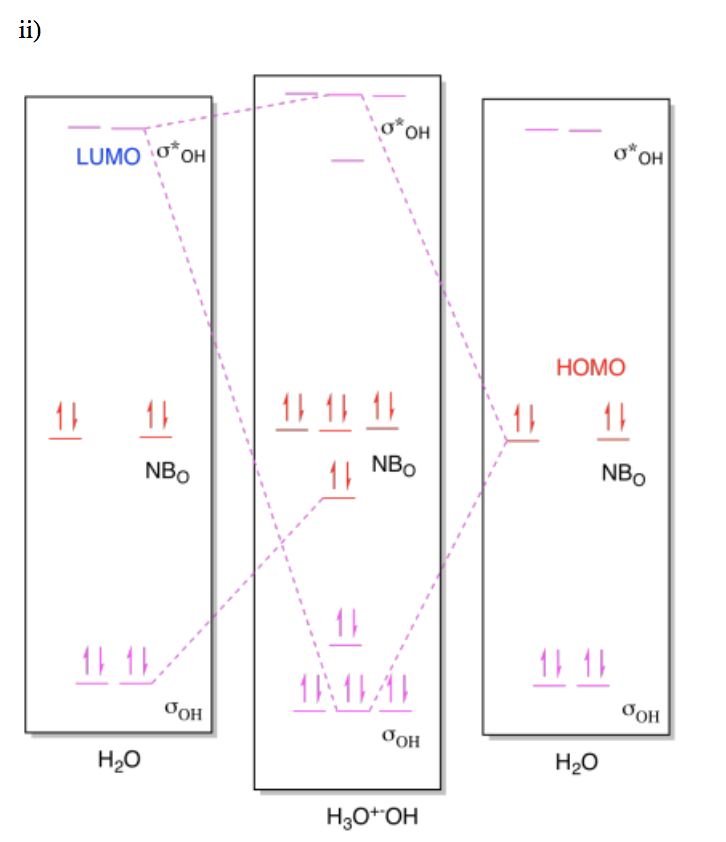
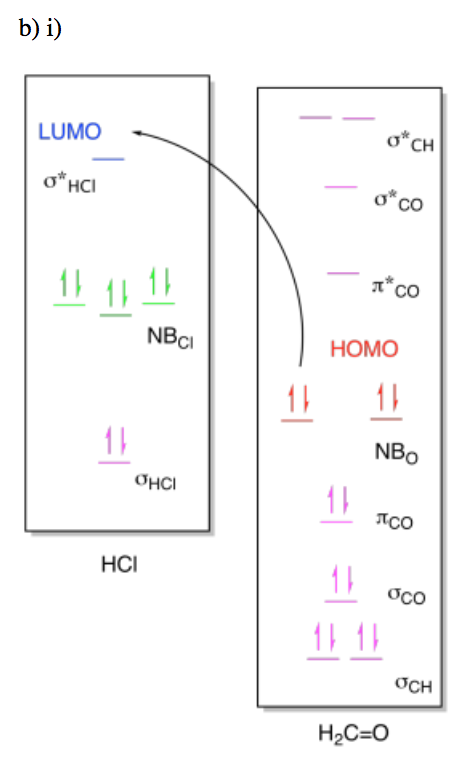
Exercise \(\PageIndex{6}\)
For the following reactions, draw (i) MO diagrams showing the HOMO-LUMO interaction and (ii) the molceular orbital interaction diagram showing the formation of the product.
- Two molecules of water react to form H3O+-OH.
- A molecule of methanal, H2CO, reacting with hydrogen chloride, HCl, to form H2COH+Cl-.
- Answer a i
-
- Answer a ii
-
- Answer b i
-
- Answer b ii
-