14.4: Lewis Acid-Base Complexes and Molecular Orbitals
- Page ID
- 193773
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)What happens when a Lewis base donates a pair of electrons to a Lewis acid? The arrow formulism we have been using to illustrate the behavior of Lewis acids and Lewis bases is meant to show the direction of electron movement from the donor to the acceptor. However, given that a bond can be thought of as a pair of electrons that are shared between two atoms (in this case, between the donor and the acceptor), these arrows also show where bonds are forming.
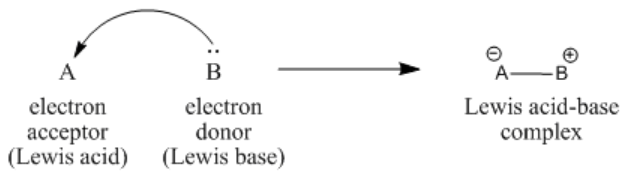
The electrons donated from a Lewis base to a Lewis acid form a new bond. A new, larger compound is formed from the smaller Lewis acid and Lewis base. This compound is called a Lewis acid-base complex.
A simple example of Lewis acid-base complexation involves ammonia and boron trifluoride. The nitrogen atom has a lone pair and is an electron donor. The boron has no octet and is an electron acceptor. The two compounds can form a Lewis acid-base complex or a coordination complex together.
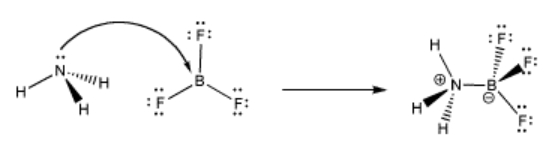
When the nitrogen donates a pair of electrons to share with the boron, the bond that forms is sometimes called a coordinate bond. Another term for this kind of bond is a dative bond. A coordinate or dative bond is any covalent bond that arose because one atom brought a pair of its electrons and donated them with another.
There is another piece of terminology you should get used to here. Sometimes, the electron donor is called a nucleophile and the electron acceptor is called an electrophile. Ammonia is a nucleophile and boron trifluoride is an electrophile.
- Because Lewis bases are attracted to electron-deficient atoms, and because positive charge is generally associated with the nucleus of an atom, Lewis bases are sometimes refered to as "nucleophiles". Nucleophile means nucleus-loving.
- Because Lewis acids attract electron pairs, Lewis acids are sometimes called "electrophiles". Electrophile meanse electron-loving.
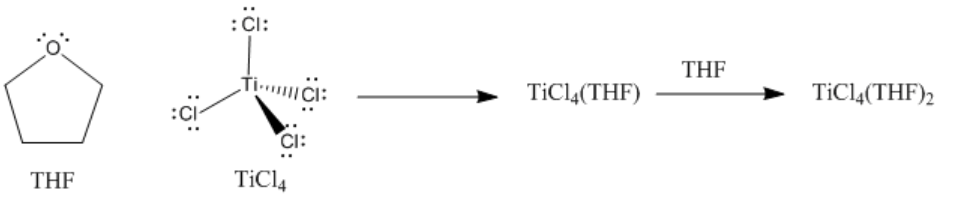
Lewis acid-base complexes frequently have very different properties from the separate compounds from which they were formed. For example, titanium tetrachloride is a yellow liquid at room temperature. It is so Lewis acidic that it reacts with moisture in the air, undergoing a reaction that generates HCl gas in the form of white smoke. Tetrahydrofuran (or THF), a mild Lewis base, is a colorless liquid. When THF and TiCl4 are combined, a Lewis acid-base complex is formed, TiCl4(THF)2. TiCl4(THF)2 is a yellow solid at room temperature. Although it still reacts with the air, it does so very slowly, and shows no visible change when exposed to the air for several minutes.
Exercise \(\PageIndex{1}\)
Use curved arrow notation to show the electron movement for the following reactions.
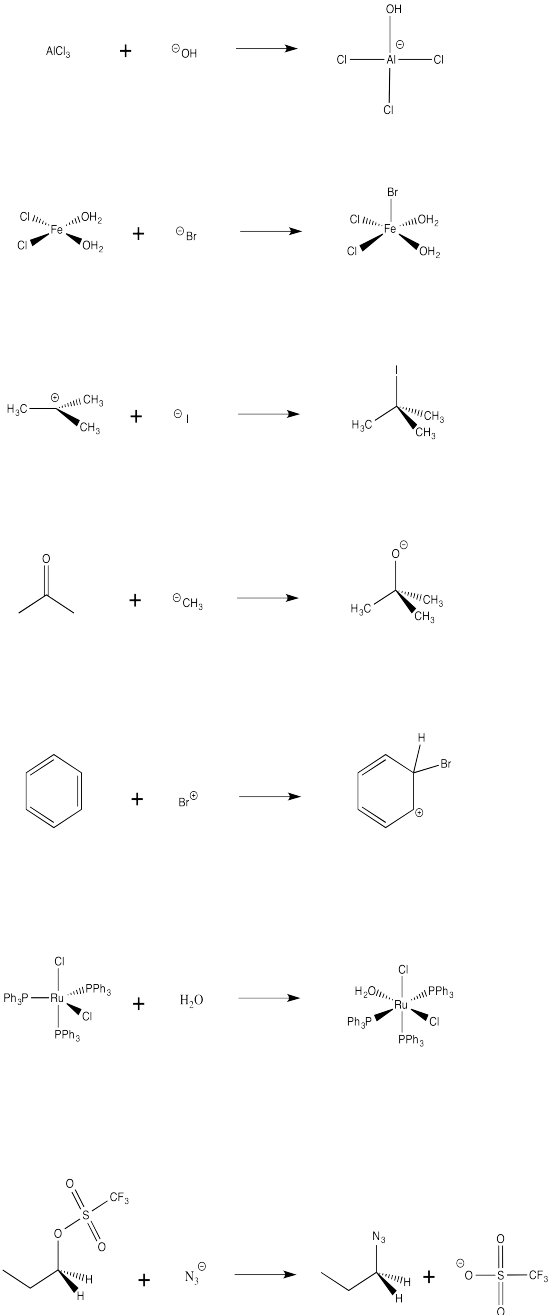
- Answer
-
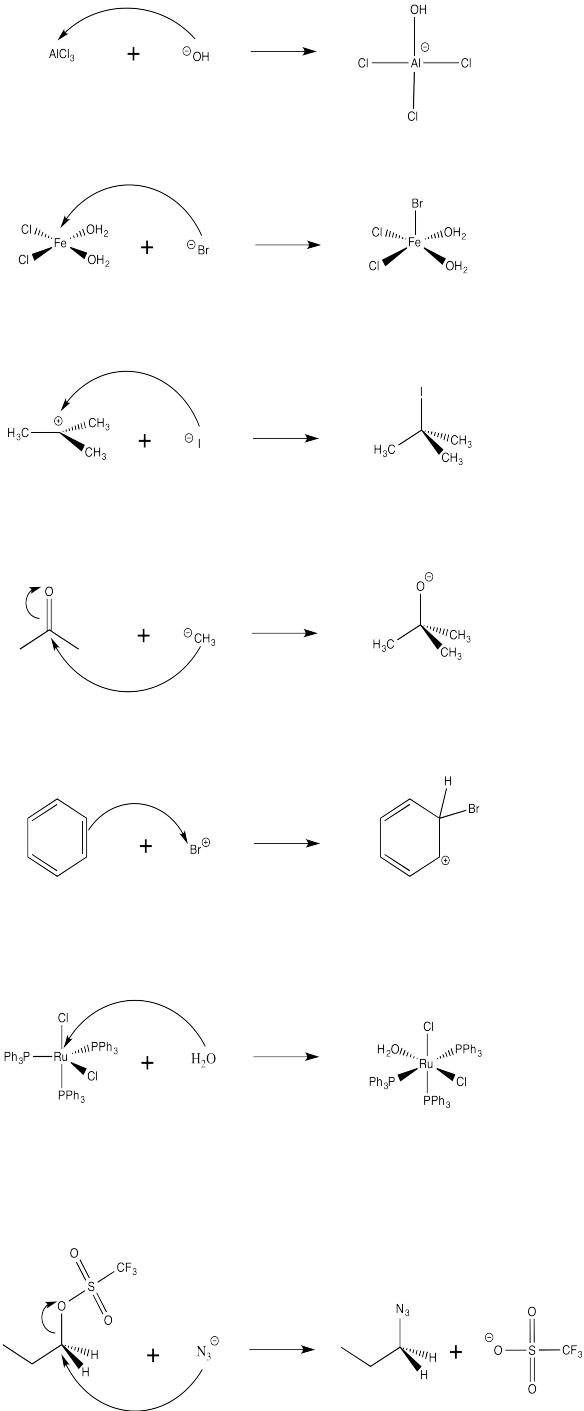
Exercise \(\PageIndex{2}\)
Show, using arrow notation, the two-step reaction between THF and titanium tetrachloride to form the Lewis acid-base complex, TiCl4(THF)2. Also show the structures of the intermediate and final complexes.
- Answer
-
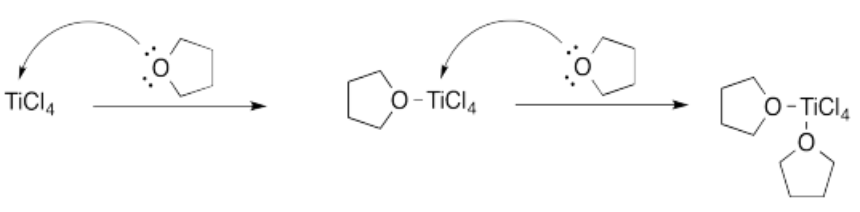
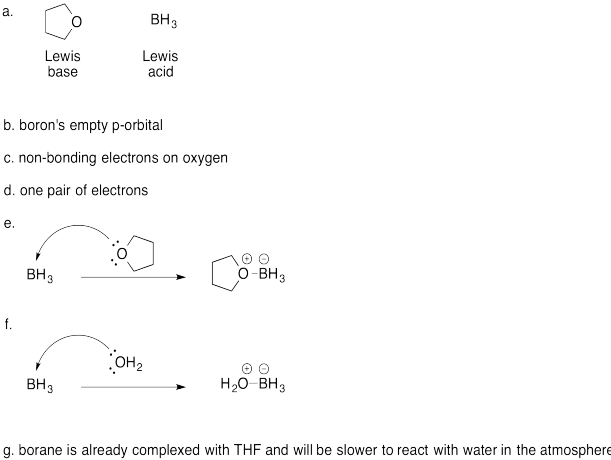
Exercise \(\PageIndex{3}\)
A similar Lewis acid-base complex is formed between THF and borane, BH3.
a) Which compound is the Lewis acid? Which one is the Lewis base?
b) Which atom in the Lewis acid is the acidic site? Why?
c) Which atom in the Lewis base is the basic site? Why?
d) How many donors would be needed to satisfy the acidic site?
e) Show, using arrow notation, the reaction to form a Lewis acid-base complex.
f) Borane is highly pyrophoric; it reacts violently with air, bursting into flames. Show, using arrow notation, what might be happening when borane contacts the air.
g) Borane-THF complex is much less pyrophoric than borane. Why do you suppose that is so?
- Answer
-
Exercise \(\PageIndex{4}\)
When a neutral Lewis acid combines with an anionic Lewis base, the product is called a complex ion. The same is true if a cationic Lewis acid combines with a neutral Lewis base.
Show the formation of the following polyatomic anions from the Lewis acid-base pairs that were combined in each case.
a) BF4- b) PF6- c) AlCl4- d) AlH4- e) Ag(NH3)2
Exercise \(\PageIndex{5}\)
Consider the reaction below.

• Draw an MO mixing diagram for the reaction above, using the following steps:
o Draw the orbital from the base that is likely to donate its electrons.
o Draw the orbital from the acid that is likely to accept electrons.
o Complete the MO mixing diagram of these two orbitals:
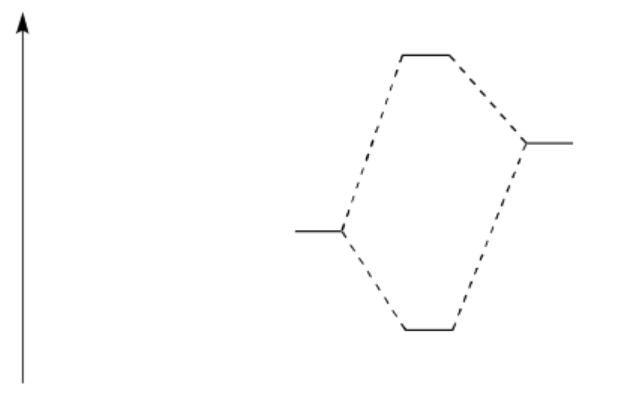
• Label the electron donating orbital
• Label the electron accepting orbital
• Populate the MO mixing diagram with electrons
o Draw a cartoon showing the mixing of these orbitals.
- Answer
-

Exercise \(\PageIndex{6}\)
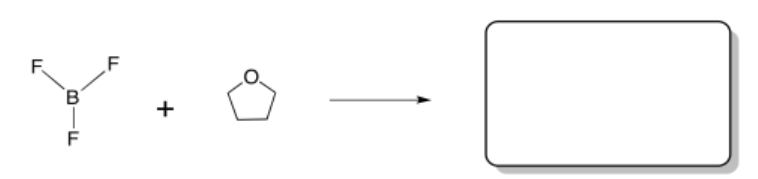
a) A typical Lewis acid/base pair is shown below.
- Label the two compounds below as either Lewis acid or Lewis base.
- Draw curved arrows to show movement of electrons to form a bond.
- Draw the product of the reaction.
b) The Lewis pair below reacts to form an adduct, but much more slowly than the one above.
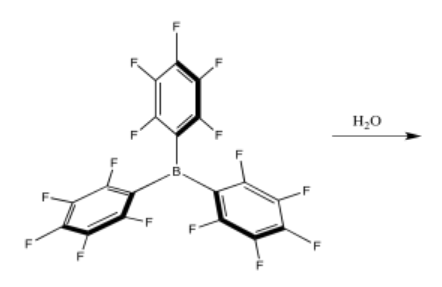
- Suggest two reasons why the Lewis acid/base adduct forms so slowly.
- Answer
-
a)
b)
- The benzene rings take up more space than a fluorine atom; they may get in the way, making it harder for the water molecule to approach.
Also, the aromatic rings may be able to form a conjugated system with the empty p orbital; if that p orbital is partially filled, the boron atom becomes less Lewis acidic.
- A larger group than fluorine may cause more steric hindrance. Replacing the two fluorines closest to the boron on each arene (aromatic ring) with a CH3 or CF3 group would slow down the formation of an adduct. Alternatively, a less electronegative group than fluorine would also make the boron seem less positive; a CH3 or OCH3 are two possibilities.
- An even more electron-withdrawing group than fluorine would make the Lewis acid more reactive. Examples include nitro (NO2) and carbonyl groups (such as CH3C=0); these groups are resonance-withdrawing. Alternatively, a smaller group such a hydrogen would lower steric resistance, but would also lead to lower electrophilicity at boron, owing to the lower electronegativity of hydrogen compared to fluorine.
Chemical reactions involve bond-making and bond-breaking events, as well as the movement of electrons. When we think about chemical reactions, we often think about where the electrons are coming from, and where they are going. In a Lewis structure picture, we most often think of the electrons as coming from a lone pair -- a non-bonding pair of electrons on one particular atom. We picture the electrons becoming attracted toward an atom that lacks electrons, maybe because it does not have a filled valence shell, or maybe because it has some amount of positive charge.
In addition to a Lewis picture, it's often useful to think about reactions in terms of molecular orbital interactions. That kind of consideration is especially useful in computational chemistry where, through the use of the right software, we can calculate energy changes that occur over the course of a reaction. It's also helpful to develop some conceptual understanding of these approaches qualitatively. This qualitative approach to molecular orbital interactions is routinely used by chemists because of the insight it can give into reactions.
One common way of thinking about reactions in this way is through the concept of frontier orbitals. This idea says that if one species is going to donate electrons to another in order to form a new bond, then the donated electrons are most likely going to come from the highest occupied energy level. In this level, called the highest occupied molecular orbital (HOMO), the electrons are further from the nucleus and therefore less tightly held by the protons in the nucleus. The electrons would be donated, in turn, to the lowest empty energy level on the other species, called the lowest unoccupied molecular orbital (LUMO).
Consider an example of such an interaction, between a hydroxide ion and a proton. A hydroxide ion, HO-, is a Lewis base. The oxygen atom has three lone pairs, any of which might be donated to a Lewis acid. A proton, H+, is a Lewis acid. For a hydrogen atom, in the very first little row of the periodic table, the "octet rule" is two electrons, so a proton would be able to accept a pair of electrons from another atom and form a covalent bond.

The atomic orbital diagram for a proton is very simple. Hydrogen has only a 1s orbital, and in H+ that energy level is empty. This orbital corresponds to the LUMO for a proton.
The molecular orbital diagram for hydroxide ion is not much more complicated. This molecule is diatomic; it comes from the combination of an oxygen atom with a hydrogem atom, with the addition of an extra electron to provide the negative charge of the ion. In the diagram below, the hydrogen atom interacts with one of the p orbitals on the oxygen, but it does not matter exactly which oxygen orbital we use.
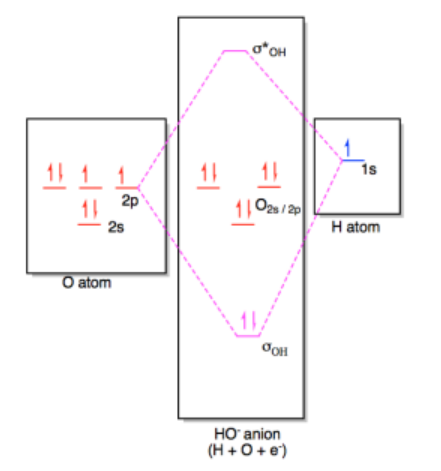
In most cases, we could come up with the MO diagram in another way. If we take the shortcut of working out an approximate MO diagram of a molecule based on its Lewis structure, and we know that hydroxide ion has one O-H bond and three lone pairs on oxygen, then we know there should be a bonding orbital at low energy, an antibonding orbital at high energy, and three non-bonding orbitals in the middle.
In a Lewis acid-base interaction, a pair of electrons would be donated from the non-bonding level on hydroxide (the HOMO) to the empty 1s orbital on the proton (the LUMO).
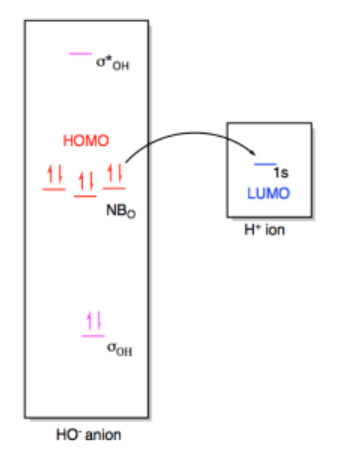
What we have here is an interaction between two orbitals. A pair of electrons in one orbital is being shared with another orbital. We already know that an interaction between two orbitals results in two new orbitals. One of the new orbitals, resulting from constructive interference, is lower in energy than either of the original orbitals. The other new orbital results from destructive interference and is higher in energy than either of the original orbitals.
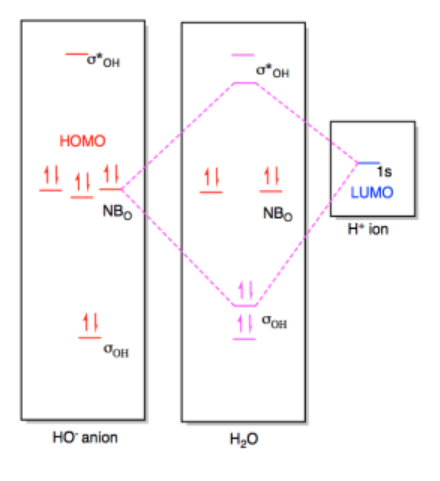
In the end, the two electrons being donated slide down in energy to become an O-H bond. The combination that rises in energy does not really matter because there are no electrons at that level, anyway. Overall, the net energy of the proton and the hydroxide ion has decreased as the pair came together to form a water molecule.
Note that the MO diagram for the resulting water molecule resempbles what we would expect from its Lewis structure. There are two low-energy O-H bonding pairs and two correspondingly high-energy antibonding orbitals. There are also two intermediate-level nonbonding pairs corresponding to the two lone pairs we see in the Lewis structure.
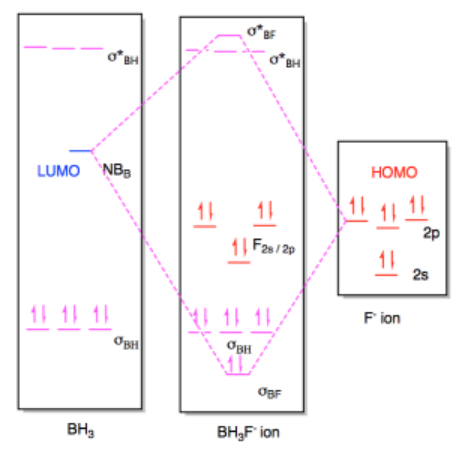
We can use the same approach to look at Lewis acid-base interaction in bigger molecules. The MO diagrams are a little busier, but the ideas are the same. For example, we could imagine a fluoride ion donating electrons to a molecule of borane, BH3. The fluoride is a Lewis base because it has lone pairs. The borane is a Lewis acid because the boron atom lacks an octet; it has only six valence electrons in its structure.

In this case, the borane contains three B-H bonds, so there will be three B-H bonding pairs and three empty B-H antibonding levels. There would be an empty orbital as well, corresponding to an empty p orbital on the boron. That empty p orbital is the lowest unoccupied molecular orbital (LUMO). A fluoride ion would have four lone pairs, and we would probably imagine a pair of electrons from one of its p orbitals as the highest occupied molecular orbital (HOMO). The interaction therefore involves donation from one of these orbitals on fluoride to the empty p orbital on borane.
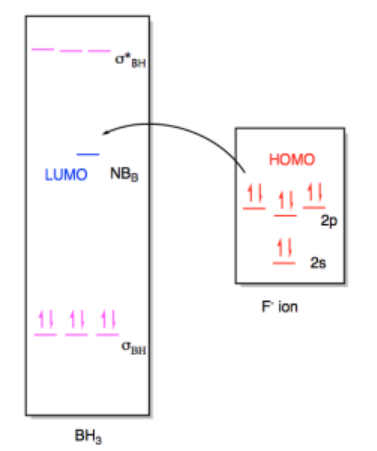
Once again, this interaction would result in a new molecule with a new molecular orbital diagram. The only appreciable changes would involve the two orbitals that interact with each other, the HOMO and LUMO. The diagram for BH3F- ion is really a superposition of the two diagrams before, except that the HOMO and LUMO have formed a new bonding and antibonding orbital for the new B-F bond.