12.9: Solutions for Selected Problems
- Page ID
- 192554
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)Exercise 12.1.1:

Exercise 12.1.2:
Exercise 12.1.4:
b) Chains of HDPE are able to pack more tightly together than LDPE, in which the branches prevent tight packing of the backbone. That difference makes LDPE less dense than HDPE.
Exercise 12.2.1:
- Water can in principle form four hydrogen bonds: it can donate two and accept two. Ethylene glycol has two OH groups, so it can donate two H bonds and accept four. That enhanced clinginess is responsible for its higher viscosity.
- Olive oil does not hydrogen bond; it isn't even very polar. However, it is a triglyceride and its fatty acid chains are about 80% oleic acid and linoleic acid, which are both 18-carbon chains. So the average olive oil molecule contains three 18 carbon branches, leading to a modest level of entanglement. Its viscosit is much higher than water's.
- Methanol has only one OH bond, so it has a little less hydrogen bonding than water, but not enough of anything else to form other interactions. Its viscosity is a little lower than water's.
- 2-propanone has no hydrogen bonding at all, although it does have a dipole moment to hold different molecules together. Its viscosity is a little lower than methanol's.
- Hexane has no dipole and no hydrogen bonding. It is really too short to become entangled. Molecules only attract each other through weak London dispersion interactions. Its viscosity is a little lower than 2-propanone's.
Exercise 12.2.2:
The greater the number of repeating units, the higher the molecular weight. Also, the greater the number of repeating units, the greater the entanglement and the higher the viscosity.
Exercise 12.3.1:
You can think about a length of chain flopping over a neighbouring strand like a grappling hook, then pulling itself along as the bond continues to rotate, vaulting over the other chain.
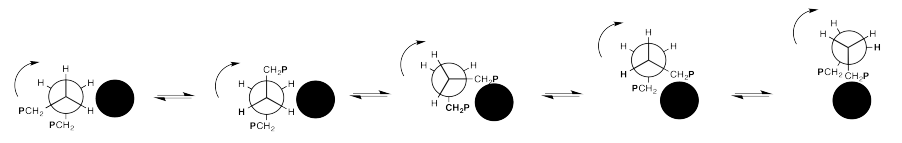
Exercise 12.3.2:
- The greater the number of repeating units, the greater the entanglement. Entanglement acts as an impediment of the movement of chains, increasing the temperature at which chain motion becomes restricted (in other words, increasing the minimum volume needed to get the chains moving again). For example, polypropylene has a higher Tg than polyethylene, and that of polystyrene is even higher (although additional factors influencethe Tg of PS).
- Increased polarity increases the attraction between chains and can even lead to formation of physical crosslinks between chains. This increased interaction hinders chains from moving freely and raises the glass transition temperature. For example, polylactide, poly(vinyl chloride) and poly(methyl methacrylate all have much higher values of Tg than polypropylene.
- If the backbone is rigid, reptation is hindered because the chain can't adopt different conformations. Chain flow is restricted more easily and the Tg is higher. Kevlar and polynorbornene both have stiff backbones because of the aromatics and aliphatic rings, respectively, that form part of the backbone. Both polymers have much higher values of Tg than polystyrene, in which the aromatics hang from a flexible chain.
Exercise 12.3.5:
a) rubbery b) glassy c) glassy d) rubbery
Exercise 12.4.1:
There may be different architectures that lend themselves to the same applications.
- A regular chain structure could be wound into a thread easily. There would be enough entanglement to held the threed together.
- Across-linked structure would help a rubber band snap back to its original shape. However, too much cross-linking would make it too difficult to stretch the rubber band.
- A dendrimer has lots of nooks and crannies that might be used to sponge up pharmaceuticals and slowly release them later.
- A branched or cross-linked structure might form a good two-dimensional network to provide a protective coating.
Exercise 12.5.1:
The polar vinyl acetate block of the first polymer contains carbonyl groups that would attract each other by dipole-dipole interactions. It is easy to imagine these segments forming physical crosslinks. The hydrocarbon chain in the middle block would prvide flexibility.
Although nonpolar, the benzene groups in styrene are flat and can stack together with relatively strong London dispersion forces. The higher Tg of polystyrene would make these blocks much stiffer than the more flexible polyisoprene block in the middle.
Exercise 12.6.1:
The telechelic polymer has two growing ends per chain, so it grows twice as fast as a normal chain.
Exercise 12.6.2:
If this chain reached 100% conversion, then the degree of polymerization would be 500. At 85% conversion, DP = 425.
Exercise 12.6.3:
As the number of repeat units increases, the molecular weight increases.
Exercise 12.7.2:
The molecular weight is the sum of the weights of all the repeat units plus the weight of the end groups.
- \(MW = 1000 units \times 104.15 \frac{Da}{unit} + 2 Da = 104152 Da\)
- \(MW = 200 units \times 72.06 \frac{Da}{unit} + 2 Da = 14412 Da\) (based on 200 simple repeating units, but this is a complicated case. In common practice, PLA is made from a dimeric monomer, with each monomer forming two repeat units in the chain, so a degree of polymerization of 200 really corresponds to 400 repeat units in the chain, which would have twice the molecular weight.)
- \(MW = 50 units \times 44.05 \frac{Da}{unit} + 2Da = 2202 Da\)
- \(MW = 100000 units \times 42.08 \frac{Da}{unit} + 2 Da = 4208002 Da\)
Note that, in this case, the end groups make no appreciable difference in the weight because they are so small.
Exercise 12.7.3:
MW = weight (in g) / moles
- 5827 Da
- 1052 Da
- 703,000 Da or 703 kDa
Exercise 12.7.4:
Đ = Mw / Mn
- 1.14
- 1.22
- 1.56
- 1.76
- 1.62
Exercise 12.7.5:
- 3,000 D
- 5,000 D
Exercise 12.7.6:
The longer the chain in polymer A, the bigger the sphere it coils into. There isn't much problem comparing samples of polymer A to each other, provided we have some way of calibrating sphere size to chain length.
However, polymer B appears to be more tightly coiled than A. A relatively small sphere could pack a lot more chain. We would have to be cautious about comparing the sphere size of B to that of A and drawing any conclusions about chain length or molecular weight.
Exercise 12.7.7:
Exercise 12.7.8:
16:1.
Exercise 12.7.9:
It's \(OH + 4 \times (CH_{2}CH_{2}O) + H.\) That's \(17 + (4 \times 44) + 1 = 18 + 176=194D\)
Exercise 12.7.10:
The ratio of c to b is 145:18 = 8:1. However, the number of hydrogens in the repeat unit c is 4; the number of hydrogens in the end group b is 2. That ratio is 2:1. That means the comparison we need is (145/4)/(18:2) = 4:1. The degree of polymerization is 4.
Exercise 12.8.1:
a) Viscosity is related to the amount of drag in the fluid. The more drag, the slower solutes will be able to move, so the diffusion coefficient is inversely related to viscosity (i.e. higher diffusion coefficientshould correlate with lower viscosity).
b) The regular chain polymer diffuses more easily than the graft copolymer / supramolecular assembly because the pendant chains in the latter lead to increased entanglement.
Exercise 12.8.2:
b) The lone pairs on the oxygens in the host would be attracted to the positively charged nitrogens in the guest. The N-H groups in the guest would even act as hydrogen bond donors to the oxygens.
c) This supramolecular assembly forms a cross-linked architecture.
d) The gel is elastic because, although it contains flexible chains and can be distorted up to a point, at some point the crosslinks pull it back to its original position.
e) When the gel is cut, probably some guests are pulled away from their hosts along the surface of the cut. WHen the edges are placed back together, the guests can find new hosts and form the cross-linked structure again.
Exercise 12.8.3:
- The palladium in the complex is Pd(II), so it is d8 and the structure of the crosslink is square planar at palladium. Ligand exchange likely occurs through an associative process. That means that before the PVP dissociates, a new ligand would bind; this ligand is probably a solvent molecule, DMSO.
- The increased crowding in the ethyl-substituted ligand, compared to the methyl, would hinder the approach of DMSO during substitution, slowing down the reaction.
- Kb is defined as ka/kd, the rate constant for the association step over the rate constant for the dissociation step. It is the binding constant for PVP-Pd formation.
- Palladium is a second row transition metal whereas platinum is a third row transition metal. Third row metals have greater ligand field splitting energies than second row metals and are generally less labile.


