12.3: Glass Transition
- Page ID
- 189684
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)Note
comprehension of this section may be aided by an understanding of conformational analysis.
Even though it appears to be a solid, a plastic bottle that you might throw into your recycling bin has some things in common with liquids. We think of the molecules in a solid as mostly static; maybe they rock back and forth a little bit, but they mostly stay put. The long chains in a polymer are constantly in motion, however.
These chains slide over each other like snakes in a process called "reptation" or "creep". This flowing motion of the chains is responsible for the flexibility of many plastics, which can often be bent easily even when they are solids.
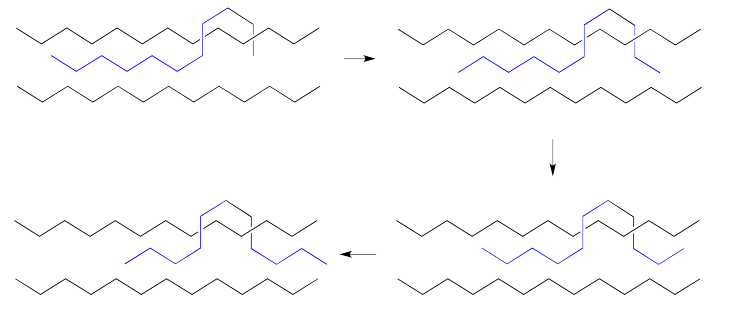
When we exert a force upon a polypropylene bottle, it changes shape. Rather than being locked together, the polypropylene chains give way and move past each other. When we let go, the chains may just slide back to their original position, or close to it, returning the bottle to its original shape.
The chains slide back because they were originally in a stable conformation. Rapidly deforming the plastic twists the chains into less favorable positions. Left alone, they twist back into positions that are energetically more favorable.
Of course, it is always possible that the plastic is deformed a little too far and does not bounce back to its original shape. In that case, the chains have become stretched out in such a way that they have become stuck. They may have become tangled in new ways. New obstacles have appeared that make it difficult for them to slide back again.
Sometimes, a force is exerted on a plastic material over a long period of time, causing the chains to slowly slide away from their original positions. The chains adopt a new equilibrium distribution. When the force is removed, the plastic has adopted a new shape. A formerly straight bar might become bowed under a weight, for example.
Exercise \(\PageIndex{1}\)
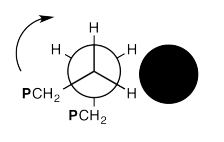
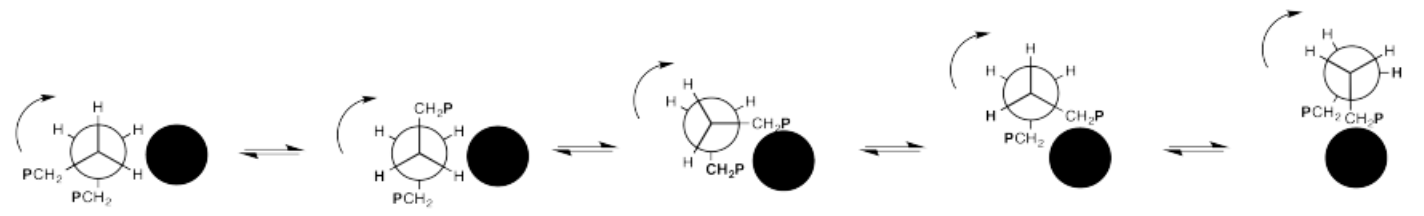
In the drawing below, we are looking down a C-C bond in a polymer chain (left). We can see hydrogens attached to the carbon in front and the carbon behind, although the carbons are not labelled. We can see the where the polymer chain is attached, labelled "CH2P". A cross-section of the neighbouring chain is also shown (in black).
Show, with the help of drawings, how a 360° rotation about that bond will cause the polymer chain on the left to creep over the one on the right.
One of the most commonly-used diagnostic tests for a polymer is the measurement of the glass transition temperature (Tg). The glass transition temperature is related to the ease of chain flow in the material.
The glass transition represents a phase change. As a polymeric material is cooled, it changes from a more flexible, rubbery state to a more rigid, glassy state. This transition occurs because of the change in volume with temperature. Generally, solids shrink as they are cooled. At some point, the volume of a polymer decreases to the point at which the chains can no longer move past each other; there just isn't enough room.
- Answer
-
You can think about a length of chain flopping over a neighbouring strand like a grappling hook, then pulling itself along as the bond continues to rotate, vaulting over the other chain.
The glass transition temperatures of a number of common polymers are listed below. These data indicate whether a given polymer will exist in a rubbery state or a glassy state at a particular temperature.
| Name | Abbreviation | Approx. Tg, °C |
| low-density polyethylene | LDPE | -120 |
| polyethylene oxide | PEO | -50 |
| polypropylene | PP | -10 |
| nylon-6 | - | 45 |
| polylactide | PLA | 65 (L isomer) |
| poly(ethylene terephthalate) | PETE | 70 |
| polyvinylchloride | PVC or V | 90 |
| polystyrene | PS or S | 100 |
| poly(methyl methacrylate) | PMMA | 110 |
| polycarbonate | PC | 145 |
| polynorbonene | - | 215 |
| kevlar | - | 240 |
These values are only approximate. The glass transition temperature actually depends on a number of factors, including the average molecular weight of the polymer and how the data is measured.
Exercise \(\PageIndex{2}\)
Explain why glass transition temperature would depend on molecular weight.
Exercise \(\PageIndex{3}\)
Glass transition temperature is influenced by several factors, including the following ones.
- sterics (crowdedness, or physical interference between groups as they move through space)
- polarity
- backbone rigidity (ability for bond rotation along backbone)
- Answer a:
-
The greater the number of repeating units, the greater the entanglement. Entanglement acts as an impediment of the movement of chains, increasing the temperature at which chain motion becomes restricted (in other words, increasing the minimum volume needed to get the chains moving again). For example, polypropylene has a higher Tg than polyethylene, and that of polystyrene is even higher (although additional factors influencethe Tg of PS).
- Answer b:
-
Increased polarity increases the attraction between chains and can even lead to formation of physical crosslinks between chains. This increased interaction hinders chains from moving freely and raises the glass transition temperature. For example, polylactide, poly(vinyl chloride) and poly(methyl methacrylate) all have much higher values of Tg than polypropylene.
- Answer c:
-
If the backbone is rigid, reptation is hindered because the chain can't adopt different conformations. Chain flow is restricted more easily and the Tg is higher. Kevlar and polynorbornene both have stiff backbones because of the aromatics and aliphatic rings, respectively, that form part of the backbone. Both polymers have much higher values of Tg than polystyrene, in which the aromatics hang from a flexible chain.
In each case, explain how the factor would influence the glass transition temperature, and choose some examples from the table to support your claim.
Exercise \(\PageIndex{4}\)
Polymers also undergo other phase transitions. For example, the melting point of polystyrene is around 240 °C. Explain, with the help of drawings, the difference between the glass transition temperature and the melting temperature.
Exercise \(\PageIndex{5}\)
Indicate whether the following materials would be found in a rubbery state or a glassy state at a comfortable room temperature, about 25°C.
a) LDPE b) PS c) PVC d) PP
- Answer a:
-
rubbery
- Answer b:
-
glassy
- Answer c:
-
glassy
- Answer d:
-
rubbery
Exercise \(\PageIndex{6}\)
Indicate whether the following materials would be found in a rubbery state or a glassy state at the boiling point of water, about 100°C.
a) PEO b) PETE c) PMMA d) PC
Exercise \(\PageIndex{7}\)
Why do you think PVC is used in household plumbing, rather than other materials such as PLA or PEO?


