11.8: Solutions for Selected Problems
- Page ID
- 192278
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
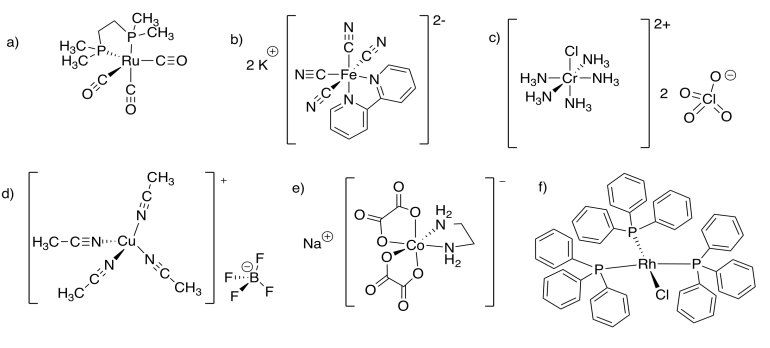
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)Exercise 11.1.1:
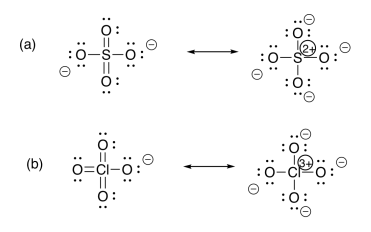
Exercise 11.1.2:
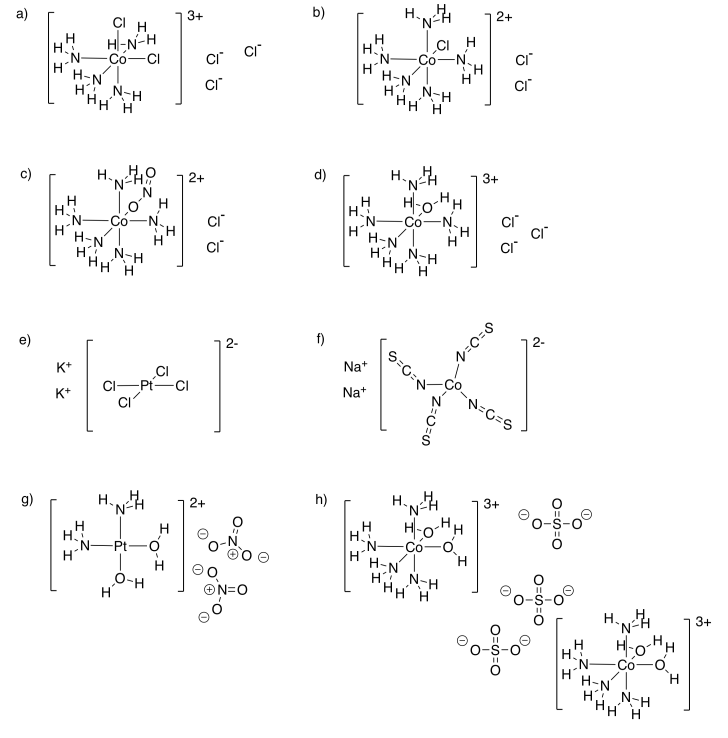
Exercise 11.2.1:
- All of these ions have the electronic configuration of the preceding noble gas, Ar. These metals have just started filling the next energy levels; these electrons are relatively high in energy, with relatively few protons attracting them (compared to later elements in the row). The valence electrons are relatively easy to use, although not the core electrons.
- These metals have relatively high numbers of protons compared to the elements to their left in the same row. They have relatively high electronegativity, so they do not lose many electrons.
- These metals in the middle display a mixture of behaviors: they are electronegative enough to stabilize relatively low charges, but far enough to the left that they can become fully oxidised to reach a noble gas configuration.
Exercise 11.2.2:
- H2O = 0 charge; Fe(II) = 2+ charge; total = 2+
- H3N = 0 charge; Cl = 1- charge; Cr(III) = 3+ charge; total = 2+
- py = 0 charge; SCN = 1- charge; Mn(II) = 2+ charge; total = 0
- CN = 1- charge; Fe(II) = 2+ charge; total = 4-
- CN = 1- charge; CO = 0 charge; Co(III) = 3+ charge; total = 2-
- CN = 1- charge; NH3 = 0 charge; Fe(II) = 2+ charge; total = 3-
Exercise 11.2.3:
- Overall charge = 3+; ligands charge = 0; charge on Cr = 3+
- Overall charge = 3-; ligands charge = 6-; charge on Fe = 3+
- Overall charge = 2+; ligands charge = 1-; charge on Cr = 3+
- Overall charge = 4-; ligands charge = 6-; charge on Mn = 2+
- Overall charge = 1+; ligands charge = 0; charge on Au = 1+
- Overall charge = 0; ligands charge = 1-; charge on Ag = 1+
Exercise 11.3.1:
a)
| Pd valence e- | 10 e- |
| charge on complex | 0 |
| charge on ligands | 0 |
| charge on Pd | 0 |
| revised Pd e- | 10 e- |
| e- donated by ligands | 4 x 2 = 8 e- |
| total | 18 e- |
b)
| Cr valence e- | 6 e- |
| charge on complex | 0 |
| charge on ligands | 0 |
| charge on Cr | 0 |
| revised Cr e- | 6 e- |
| e- donated by ligands | 6 x 2 = 12 e- |
| total | 18 e- |
c)
| Cu valence e- | 11 e- |
| charge on complex | +1 |
| charge on ligands | 0 |
| charge on Cu | +1 |
| revised Cu e- | 10 e- |
| e- donated by ligands | 4 x 2 = 8 e- |
| total | 18 e- |
d)
| Fe valence e- | 8 e- |
| charge on complex | 0 |
| charge on ligands | 0 |
| charge on Fe | 0 |
| revised Fe e- | 8 e- |
| e- donated by ligands | 5 x 2 = 10 e- |
| total | 18 e- |
e)
| Fe valence e- | 8 e- |
| charge on complex | -4 |
| charge on ligands | -6 |
| charge on Fe | +2 |
| revised Fe e- | 6 e- |
| e- donated by ligands | 6 x 2 = 12 e- |
| total | 18 e- |
Exercise 11.3.2:
a)
| Rh valence e- | 9 e- |
| charge on complex | 0 |
| charge on ligands | -3 |
| charge on Rh | +3 |
| revised Rh e- | 6 e- |
| e- donated by ligands | 5 x 2 = 10 e- |
| total | 16 e- |
b)
| Ni valence e- | 10 e- |
| charge on complex | 2+ |
| charge on ligands | 0 |
| charge on Ni | 2+ |
| revised Ni e- | 8 e- |
| e- donated by ligands | 4 x 2 = 8 e- |
| total | 16 e- |
c)
| Cu valence e- | 11 e- |
| charge on complex | 2+ |
| charge on ligands | 0 |
| charge on Cu | 2+ |
| revised Cu e- | 9 e- |
| e- donated by ligands | 4 x 2 = 8 e- |
| total | 17 e- |
d)
| Pt valence e- | 10 e- |
| charge on complex | 2- |
| charge on ligands | 6- |
| charge on Pt | 4+ |
| revised Pt e- | 6 e- |
| e- donated by ligands | 6 x 2 = 12 e- |
| total | 16 e- |
Exercise 11.4.1:
- en = bidentate; OH = monodentate
- dmpe = bidentate; bpy = bidentate; Cl = monodentate
- acac = bidentate; H2O = monodentate
- ox = bidentate; H20 = monodentate
- dmpe = bidentate; H = monodentate
- H = monodentate; PPh3 = monodentate; OAc = bidentate. In this case, the acetate gets the complex to 18 electrons by binding twice.
- en = bidentate; Cl = monodentate
- bpy = bidentate; HOCH2CH3 = monodentate
Exercise 11.4.2:
- Overall charge = 1-; ligands charge = 4-; charge on Cr = 3+
- Overall charge = 1-; ligands charge = 3-; charge on Mn = 2+
- Overall charge = 1+; ligands charge = 2-; charge on Cr = 3+
- Overall charge = 1+; ligands charge = 2-; charge on Co = 3+
- Overall charge = 3-; ligands charge = 6-; charge on Mn = 3+
- Overall charge = 3-; ligands charge = 6-; charge on Cr = 3+
- Overall charge = 1-; ligands charge = 2-; charge on Au = 1+
Exercise 11.4.3:
- Cr = d6; Cr3+ = d3; ox = 2 x 4e- = 8 e-; water = 2 x 2e- = 4 e-; total = 15 e-
- Mn = d7; Mn2+ = d5; acac = 3 x 4e- = 12 e-; total = 17 e-
- Cr = d6; Cr3+ = d3; en = 2 x 4e- = 8 e-; Cl = 2 x 2e- = 4 e-; total = 15 e-
- Co = d9; Co3+ = d6; en = 2 x 4e- = 8 e-; OH = 2e-; Cl = 2 e-; total = 15 e-
- Mn = d7; Mn3+ = d4; ox = 3 x 4e- = 12 e-; total = 16 e-
- Cr = d6; Cr3+ = d3; ox = 3 x 4e- = 12 e-; total = 15 e-
- Au = s1d10; Au1+ = d10; bpy = 2 x 2e- = 4 e-; cyanide = 2 x 2e- = 4 e-; total = 18 e-
Exercise 11.5.1:
Exercise 11.5.2:
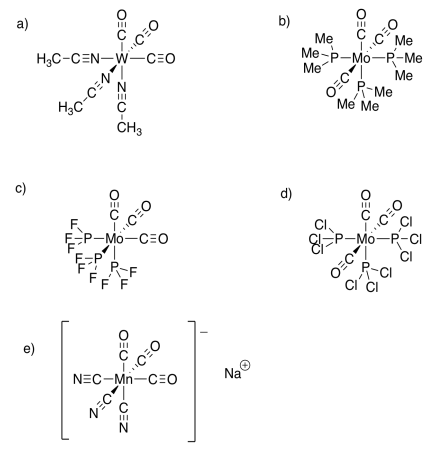
Exercise 11.6.1:
a) potassium diaquabis(oxalato)chromate(III) b) pentaamminebromocobalt(III) nitrate c) dichlorobis(ethylenediamine)chromium(III) hexafluorophosphate
d) bis(bipyridine)chlorohydroxocobalt(III) perchlorate e) triaquatrichlorotitanium(III) f) potassium hexacyanoferrate(III)
g) sodium bipyridinedicyanoaurate(I)
Bipyridine is sometimes called "bipyridyl".
Exercise 11.6.2: