11.6: Naming Transition Metal Complexes
- Page ID
- 189680
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)It's sometimes useful to be able to be able to read the name of a compound and understand what that means. The names of coordination compounds tend to be pretty long because of the number of pieces involved, but they usually follow a trend.
- Coordination compounds often contain a "complex" ion, with ligands attached to the metal ion, as well as a counterion, to balance charge. If there is a cation and an anion, the cation is named first.
[Ni(OH2)6]Cl2, hexaaquanickel(II) chloride (nickel cation before chloride anion)
In the formula, you can always tell one ion from the other because the complex ion (metal plus ligands) is always enclosed in square brackets. Anything outside of brackets is the counterion or counterions.
- Ligands are listed in front of the metal.
[Cu(NH3)6]Br2, hexaamminecopper(II) bromide (ammine ligand before copper metal)
Note that anionic ligands generally end in "o" in the name of a coordination compound. Chloride becomes chloro. Acetate becomes acetato. Neutral ligands keep their regular names, but the two common molecules H2O and NH3 are called "aqua" and "ammine", respectively.
If two different ligands are found in the complex, they are listed alphabetically.
[Co(OH2)5Cl](NO3)2, pentaaquachlorocobalt(III) nitrate (aqua before chloro)
- If there is more than one ligand of the same kind, then the number of ligands is indicated as follows:
ligands prefix 2 di 3 tri 4 tetra 5 penta 6 hexa
In the examples above, there were six waters, "hexaaqua", six ammines, "hexaammine", and five waters, "pentaaqua".
Sometimes ligand names are complicated. For example, they may already contain prefixes denoting a number, such as ethylenediamine, NH2CH2CH2NH2. The di means that there are two amino groups in the ligand. We avoid saying "di" to describe something that already contains a "di". Instead, for more complicated ligands like this, we use the following system:
| ligands | prefix |
| 2 | bis |
| 3 | tris |
| 4 | tetrakis |
| 5 | pentakis |
| 6 | hexakis |
For example, [(Ph3P)4Pd] is tetrakis(triphenylphosphine)palladium(0).
If you need to stress that only one ligand of a certain type is present, you could use the numbering adjective "mono". For example, if you are comparing a compound with two CO ligands to another that has only one, you might call them "the dicarbonyl compound" and "the monocarbonyl compound", respectively.
- The metal is named following the ligands, and its charge or oxidation state is listed using Roman numerals.
[Co(en)2Cl2]ClO4, dichlorobis(ethylenediamine)cobalt(III) perchlorate.
However, if the complex ion containing the metal is an anion, the metal is given the suffix "-ate", sometimes replacing its normal ending.
K2[PtCl6], potassium hexachloroplatinate(IV)
If the complex ion is an anion and the element's symbol does not match its name in English, the Latin name of the metal is usually used rather than the English one. For example, an iron-containing anion would contain the term "ferrate" instead of "iron"; a copper-containing one would be called a "cuprate", one with gold would be called an "aurate" and so on.
- There is no need to describe the number of counterions, because that information can be worked out given the charge on the metal and the charges on the ligands.
Na2[CuF4], sodium tetrachlorocuprate(II) (no need to say there are two sodium ions).
Exercise \(\PageIndex{1}\)
Provide names for the following complexes:
a) K[Cr(ox)2(OH2)2] b) [Co(NH3)5Br](NO3)2 c) [Cr(en)2Cl2]PF6 d) [Co(bpy)2(OH)Cl]ClO4
e) [TiCl3(OH2)3] f) K3[Fe(CN)6] g) Na[Au(bpy)(CN)2]
- Answer a:
-
potassium diaquabis(oxalato)chromate(III)
- Answer b:
-
pentaamminebromocobalt(III) nitrate
- Answer c:
-
dichlorobis(ethylenediamine)chromium(III) hexafluorophosphate
- Answer d:
-
bis(bipyridine)chlorohydroxocobalt(III) perchlorate
- Answer e:
-
triaquatrichlorotitanium(III)
- Answer f:
-
potassium hexacyanoferrate(III)
- Answer g:
-
sodium bipyridinedicyanoaurate(I)
Bipyridine is sometimes called "bipyridyl".
Exercise \(\PageIndex{2}\)
Draw the following structures:
- dicarbonylbis(1,2-dimethylphosphino)ethaneruthenium (0)
- potassium bipyridyltetracyanoferrate(II)
- pentaamminechlorochromium(III) perchlorate
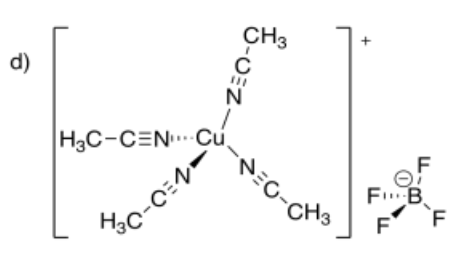
- tetraacetonitrilecopper(I) tetrafluoroborate
- sodium ethylenediaminebis(oxalato)cobalt(III)
- chlorotris(triphenylphosphine)rhodium(I)
- Answer a:
-
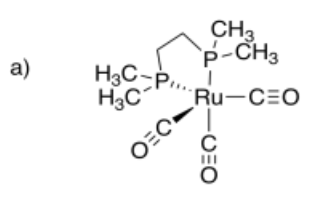
- Answer b:
-
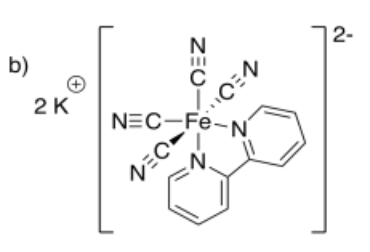
- Answer c:
-
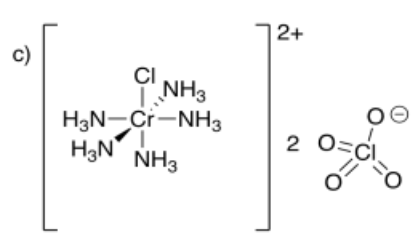
- Answer d:
-
- Answer e:
-
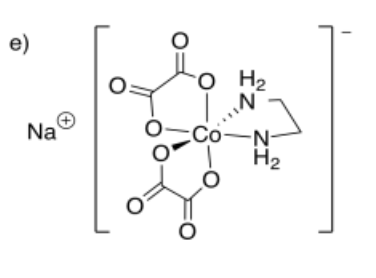
- Answer f:
-
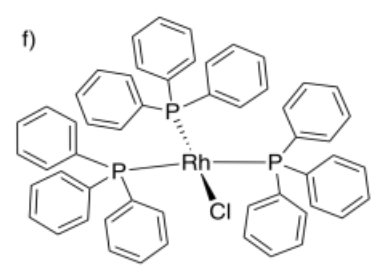
See a more in-depth discussion of coordination complexes in a later course.


