7.15: Solutions to Selected Problems
- Page ID
- 192018
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)Exercise 7.1.1:
One difference between water and these other molecules is that water is polar: there is a significant electronegativity difference between the oxygen and the hydrogen. The charges in one water molecule may be interacting with charges in other water molecules.
Exercise 7.2.1:

Exercise 7.3.1:
As far as we can tell from these data, the melting point of nitrogen and oxygen would be somewhere around -220°C, whereas the boiling point would be around -180°C. We would expect these numbers to be similar to fluorine, which has a similar mass. In fact, the melting and boiling points of oxygen are about -219°C and -183°C, respectively. The melting and boiling points of nitrogen are about -210°C and -196°C, respectively.
Exercise 7.4.1:
- A chain that is 8 carbons long would have much lower London interactions than a chain that is 102 atoms long. The greater the contact between molecules, the greater the chance for weak attractions due to small charges arising from random electron movement. The molecule with the longer chain would have the higher melting point, because more energy would have to be added in order to overcome those attractions and get the molecules moving.
- The first chain is more linear whereas the second chain is more branched. The more linear molecules will pack together more easily, allowing greater surface contact for increased London interactions. The straighter chain would have a higher melting point.
- Bromomethane has a lot more electrons than chloromethane. Based on London interactions alone, we would expect bromomethane to have a higher melting point. In fact, the melting point of bromomethane is about -94°C, compared to about -97°C for chloromethane; there must be another factor that compensates for the difference in numbers of electrons and makes these values so close.
Exercise 7.4.2:
Because of the large numbers of electrons in bromine, we would expect a higher boiling point in 3,4-dibromohexane.
Exercise 7.4.3:
Remember benzyl refers to the group C6H5CH2 (below right); cyclohexylmethyl suggests the superficially similar C6H12CH2 (below left).

The benzyl group contains trigonal planar carbons, whereas the cyclohexylmethyl group contains only tetrahedral carbons. The benzyl groups,being flatter, could pack more easily together and interact more strongly. We would expect dibenzyl ether to have a higher melting point.
Exercise 7.5.1:
These compounds all contains lots of non-polar C-C and C-H bonds, which would not have appreciable dipoles. However, in each pair, there is a compound that contains more polar bonds as well: C-Cl, C-N and C-O.
Exercise 7.5.2:
Dipoles are vectors. If we have two polar bonds, they will add together using vector addition. Any vector can be shown as the sum of two other vectors. Below, the red arrow points down and to the left. How far down and how far to the left? We can show how far using the blue arrows. The blue arrow down and the blue arrow to the left add up to give the red arrow that goes down and to the left.

In dichloromethane, there are two polar bonds. Both bonds are polarized towards the more electronegative chlorine. As drawn below, one bond has a dipole straight to the right. The other bond has a dipole that goes down and to the left. If the molecule has an overall dipole, with a positive end of the whole molecule and a negative end of the whole molecule, which end would be positive and which would be negative?

It seems reasonable that the positive end would be somewhere over on the hydrogen side and the negative end would be somewhere over on the chlorine side. The overall dipole, as opposed to the individual bond dipoles, would point somewhere in the direction of the blue, dashed arrow. But how big would it be? Surely it's bigger than either of the two individual dipoles, because they are adding together, right?
Let's take a very qualitative look. The first red arrow is just to the right. We'll break the second arrow into a smaller arrow down and an even smaller arrow to the left. The sum of the left/right arrows is a very small arrow to the right.
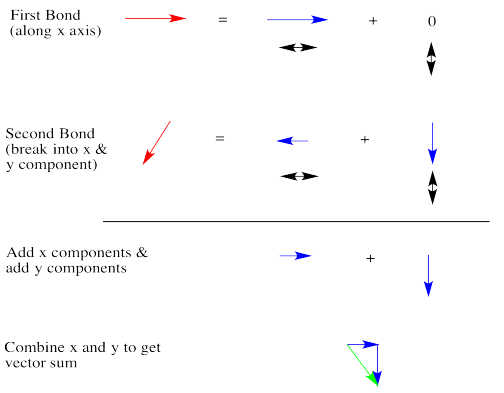
The sum of the up/down arrows is just a small arrow down. Overall, the sum is a green arrow going down and to the right. The result does not really seem any bigger than an individual bond dipole.
Exercise 7.5.3:
Despite the relatively similar dipole moments, dichloromethane's boiling point is much higher than chloromethane's. The difference probably lies in the much greater mass of dichloromethane.
Exercise 7.5.4:
In each case, the one on the right has a larger dipole and a higher melting point.

Exercise 7.6.1:
Only ethylamine and ethanoic acid have the N-H or O-H bonds that are polar enough for hydrogen bonding.
Exercise 7.6.2:
hexylamine > dipropylamine > triethylamine
The one with the greatest potential for hydrogen bonding will have the highest melting point.
Exercise 7.6.3:
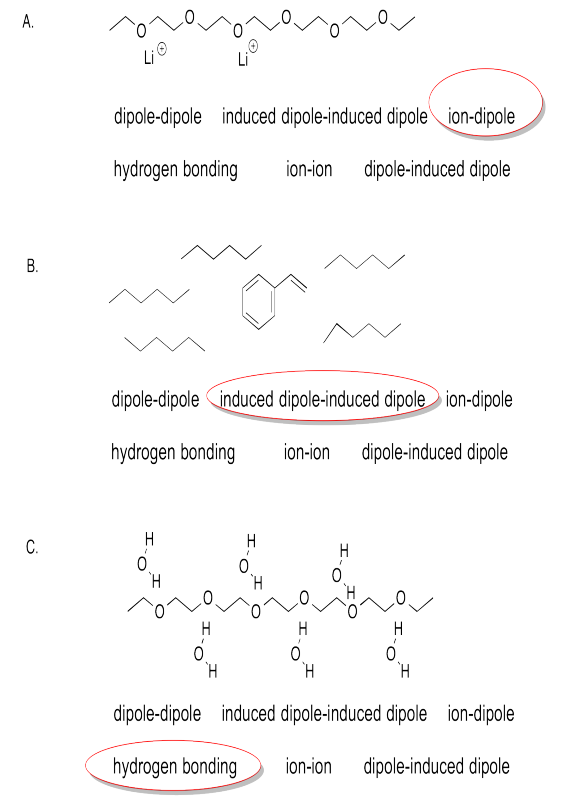


Exercise 7.8.1:
The hydrogen bonding between 2-propanol molecules is a stronger interaction and is more difficult to overcome than the dipole-dipole interactions between 2-propanone molecules. Thus, 2-propanone molecules can more easily escape into the vapour phase.
Exercise 7.8.2:
There is hydrogen bonding between molecules of N-methylethylamine, but not between molecules of triethylamine, which are held together by relatively weak dipoles. Trimethylamine molecules are not held together as tightly as the N-methylethylamine molecules, which therefore pack more densely. On the other hand, n-propylamine contains two N-H bond, compared to only one in N-methylethylamine. Consequently, n-propylamine has a greater propensity to form hydrogen bonds, and it packs together even more densley than the N-methylethylamine..
Exercise 7.8.3:
Temperature is a measure of how much energy is available in the environment. As temperature increases, molecules obtain more energy and they transfer that energy into a variety of molecular motions. As they move around more, they are less able to remain densely packed. The density decreases as the temperature goes up. Conversely, things tend to shrink as they cool, because molecules slow down and begin to pack more tightly. They become more dense.
Exercise 7.8.4:
- Acetic acid posesses hydrogen bonding capability, whereas 2-propanone has the ability to form only dipole-dipole interactions. Thus, 2-propanone molecules are not held in the liquid phase as strongly as acetic acid, and the former compound has a higher vapour pressure.
- The C=O bond of ethyl acetate has a substantial dipole, but the dipoles in ether don't add up to very much; they are smaller to begin with and they partially cancel out via vectorial addition. Ether molecules can escape the liquid more easily, resulting in a higher vapour pressure.
- Both molecules are limited to relatively weak London dispersion interactions. Because heptane forms a longer chain than pentane, there will be a slightly greater interaction between heptane molecules than between pentane molecules. Pentane thus has the higher vapour pressure.
Exercise 7.8.5:
- The C=O bond 2-butanone has a substantial dipole, but the dipoles in ether don't add up to very much; they are smaller to begin with and they partially cancel out via vectorial addition. The 2-butanone molecules cling together more strongly, resulting in a higher viscosity.
- Both molecules are limited to relatively weak London dispersion interactions. Because decane forms a longer chain than hexane, there will be a slightly greater interaction between decane molecules than between hexane molecules. Decane thus has the higher viscosity. Later, if you study macromolecules, you will see that in very, very long chains, "entanglement" becomes a factor as well. Just as the name implies, really long chains get tangled up together and have a difficult time moving past each other; that's why materials like cooking oil and motor oil are so viscous.
- The 1-butanol can hydrogen bond together, but the ether only has weak dipole-dipole interactions. The 1-butanol is therefore more viscous.
Exercise 7.8.6:
- The 1-butanol can hydrogen bond together, but the ether only has weak dipole-dipole interactions. The 1-butanol therefore has greater surface tension.
- The 1-butanol can hydrogen bond together, but the 1,3-butanediol has two OH groups and can form even more hydrogen bonds than 1-butanol can. The 1,3-butanediol therefore has greater surface tension.
- The formamide has N-H bonds and so it is capable of hydrogen bonding. Despite having a pretty darned big dipole, the nitromethane can't really hydrogen bond together. The formamide therefore has greater surface tension.
Exercise 7.8.7:
- 2-propenol. It has hydrogen bonding capability but propanal does not.
- heptanoic acid. It has hydrogen bonding capability but ethyl pentanoate does not.
- 1-hexen-3-one. Its dipole is greater than that in the ether, because it comes from a C=O bond rather than C-O bonds.
Exercise 7.8.8:
- 2-butanone. Its dipole is greater than that in the ether, because it comes from a C=O bond rather than C-O bonds.
- octane. It has greater London dispersion interactions that pentane because of the greater surface contact area in octane.
- 1,3-butanediol. It has hydrogen bonding but the DME does not.
Exercise 7.8.9:
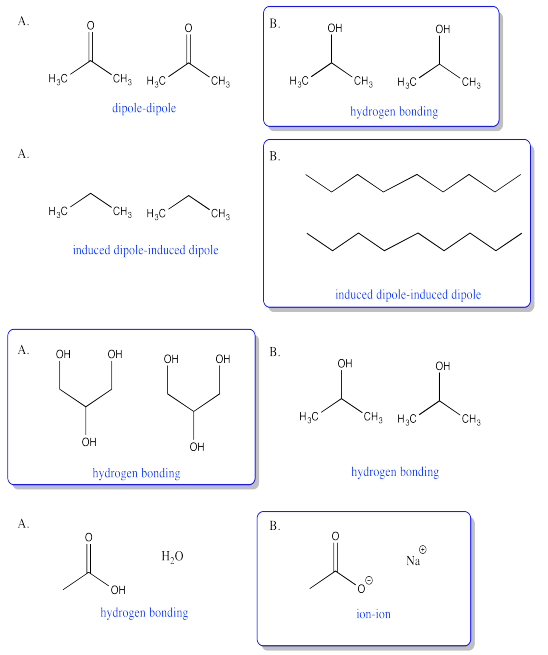

Exercise 6.9.1:
Hydrogen bonding.
Exercise 6.9.2:
a) dipole interactions b) London interactions c) dipole interactions d) hydrogen bonding
Exercise 6.9.3:
Water molecules interact with each other mainly through hydrogen bonding, whereas octane molecules interact with each other via London interactions. The main problem here is the strong hydrogen bonding between the water molecules. All molecules, in principle, could interact with each other via London interactions. However, if octane molecules were introduced among the water molecules, they would take up space. Some of the water molecules would not be able to get close enough to each other to hydrogen bond anymore. The loss of that very stabilizing interaction would be too costly.
Exercise 6.9.4:
This problem is similar to the previous one, but in this case the attraction between the strong dipoles of the nitrile groups would be too much to overcome.
Exercise 6.9.5:


Exercise 7.9.6:
a) dipole / induced dipole b) dipole / dipole c) hydrogen bonding d) dipole / induced dipole
Exercise 7.10.1
The predicted order, from most soluble to least, would be LiCl > MgSO4 > AlPO4 because the ions increase in charge from 1+/- to 2+/- to 3+/-
Exercise 7.10.2:
The predicted order, from most soluble to least, would be dimethylsulfoxide > acetonitrile > pyridine > dichloromethane > triethylamine.
Exercise 7.11.1:
a) H-bond acceptor b) H-bond acceptor c) fully H-bonding d) H-bond acceptor e) fully H-bonding f) neither
Exercise 7.12.1:


Exercise 7.13.1:
Note that the last example is actually found in RNA, not DNA. RNA is found as a single strand, rather than a pair of strands. It hydrogen bonds to itself in a particular shape, not unlike the way a protein adopts a specific shape.

Exercise 7.13.2:

Exercise 7.13.3:
Although lignin is capable of some hydrogen bonding, it cannot make nearly as many hydrogen bonds as cellulose can. Paper containing lignin would be much weaker, and more likely to tear or to fall apart when it gets wet, because the cellulose molecules would not be able to bind each other as tightly with lignin mixed in. In fact, brown paper bags from the grocery store are made from kraft, which has not had lignin removed; they are much weaker than most paper, especially if they get wet.

Exercise 7.13.4:
The dominant intermolecular attraction here is just London dispersion (or induced dipole only). London dispersion is very weak, so it depends strongly on lots of contact area between molecules in order to build up appreciable interaction. The stearic acid-containing triglycerides will be able to pack much more closely together than the more jumbled oleic acid-containing ones, so they will have much more surface contact and much stronger attractions. they will hold together into a solid.
Exercise 7.13.5:
a) alpha helix b) beta sheet c) alpha helix d) beta sheet e) beta sheet
f) alpha helix g) alpha helix h) beta sheet i) alpha helix j) alpha helix
Exercise 7.14.1:
a)
| water | water | |
| ionic | no | no |
| H-bond acceptor | yes | yes |
| H-bond donor | yes | yes |
| dipole | yes | yes |
| induced dipole | yes | yes |
Water hydrogen bonds with water.
b)
| goof | goof | |
| ionic | no | no |
| H-bond acceptor | no | no |
| H-bond donor | no | no |
| dipole | no | no |
| induced dipole | yes | yes |
Goof-off forms induced dipole/induced dipole interactions (London dispersion forces) with itself.
c)
| goof | grease | |
| ionic | no | no |
| H-bond acceptor | no | no |
| H-bond donor | no | no |
| dipole | no | no |
| induced dipole | yes | yes |
Goof-off forms induced dipole/induced dipole interactions (London dispersion forces) with grease.
d)
| grease | water | |
| ionic | no | no |
| H-bond acceptor | no | yes |
| H-bond donor | no | yes |
| dipole | no | yes |
| induced dipole | yes | yes |
Water forms dipole/induced dipole interactions with grease.
The key to why goof-off works is that it engages in the same London dispersion interactions with the grease as the grease does itself. Water is good at dissolvng many things, but not grease. That's mostly because the water would have to give up some of the strong hydrogen bonds with other water molecules in order to fit grease molecules among the water molecules.
Exercise 7.14.2:
a)
| octane | octane | |
| ionic | no | no |
| H-bond acceptor | no | no |
| H-bond donor | no | no |
| dipole | no | no |
| induced dipole | yes | yes |
That's going to be induced dipole-induced dipole interactions in octane.
b)
| isopropanol | isopropanol | |
| ionic | no | no |
| H-bond acceptor | yes | yes |
| H-bond donor | yes | yes |
| dipole | yes | yes |
| induced dipole | yes | yes |
Hydrogen bonding in isopropanol.
c)
i)
| isopropanol | water | |
| ionic | no | no |
| H-bond acceptor | yes | yes |
| H-bond donor | yes | yes |
| dipole | yes | yes |
| induced dipole | yes | yes |
Hydrogen bonding between isopropanol and water.
ii)
| octane | isopropanol | |
| ionic | no | no |
| H-bond acceptor | no | yes |
| H-bond donor | no | yes |
| dipole | no | yes |
| induced dipole | yes | yes |
Dipole-induced dipole interactions between octane and isopropanol.
d)
e) No, the ethanol should do the same thing that the HEET does.
Exercise 7.14.3:
a)

b) The amphiphile inserts into the cell membrane. Draw a cartoon of a portion of a cell membrane. Add a cartoon of the synthetic amphiphile to the membrane. What is the IMF through which it interacts with the membrane?
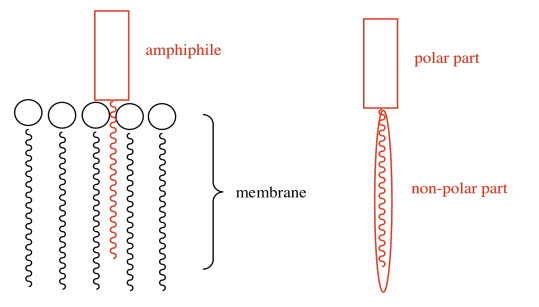
c) Ca2+ (two atoms from the left edge).
d) PO43- because \( 3 \times (2^{+}) + 2 \times (3^{-}) = 0\); neutral charge overall.
e) These high charges (2+/3-) bind much more tightly than the low charges (1+/1-) in sodium chloride.
f)

Exercise 7.14.4:
a)

b)

c)

d)

e) The carboxylic acid group provides sites for hydrogen bonding, including both a hydrogen bond donor in the OH group and an additional hydrogen bond acceptor in the C=O group.
f) It introduces a "hydrophobic" part in which the major intermolecular force with water would be a dipole-induced dipole interaction. If the water molecules keep to themselves, they will be able to maximize the amount of hydrogen bonding they can do with each other. The water is better off not mixing in with the paper.
g) All proteins have polar backbones formed by their amide bonds or peptide linkages (-NH-C=O). These portions can hydrogen bond with the cellulose in the paper. In addition, there may be polar side chains such as serine or threonine, for example, containing OH groups that can hydrogen bond with the paper.
h) Hydrophobic residues would add water resistance, for the same reason as the hydrophobic part of the abietic acid in rosin.
i) Ti4+ would have the same electronic configuration as argon. O2- would have the same electronic configuration as neon.
j) The formula is TiO2, so that the positive charges (4+) and the negative charges (2 x 2- = 4-) are balanced.
k) The 4+ and 2- charges are pretty large. Highly charged ions are difficult to dissolve, because the ion-dipole attraction of the ion with the water cannot compete with the very strong ionic attractions between the ions.
l) Ion-dipole. You can imagine the partially negative oxygen atoms of cellulose interacting with the titanium cations and the partially positive hydrogens of the OH groups in the cellulose interacting with the oxide anions.
m) Ti: one full atom in the center and eight in the corners, so 1 + 8x(1/8) = 2 Ti atoms.
O: two full atoms (towards the left and right ends of the picture) plus four half atoms (on the upper and lower faces of the cell), so 2 + 4x(1/2) = 4 O atoms.
ratio: 1 Ti : 2 O or TiO2 in formula notation.
n) The Ti are packed in a cell that resembles a body-centered cubic cell. However, the cell is not really cubic, because it is stretched out a little. Sometimes we call that shape "tetragonal".
o) The benzyl alcohol could interact with the eosin in a number of ways, because eosin is a fairly large, complicated molecule (at least at this stage in your studies). We could imagine ion-dipole interactions, in which the partially positive hydrogens on the OH groups in the benzyl alcohol interact with the anionic oxygens of the eosin, or the partially negative oxygens in the benzyl alcohol interact with the positive calcium ion.

However, the polar part of benzyl alcohol is really the smaller part of the molecule. The bigger part of the molecules is the benzene, a six-membered carbon ring with three double bonds. The carbons at the ends of those double bonds are trigonal planar, so because the benzene is built up from planar atoms, it is completely flat. C-C bonds are not polar, and C-H bonds are hardly polar at all, so the dominant IMF in this part of the molecule is London dispersion or induced dipole-induced dipole interaction. That's a pretty weak interaction generally, but it can get a little stronger between large molecules, or between flat ones, because two flat molecules can have more surface area in contact with each other. That means the benzenes in benzyl alcohol and the benzenes in eosin have appreciable London forces that allow them to interact with each other.
p) With the cellulose, covered in OH groups, the polar interactions outlined above will become more prominent.
Exercise 7.14.5:
- OAc is an ester.
- AAI has three chiral centers; GATA has five.
- The chains in the adhesive would stick to each other via intermolecular attractions. Dipole-dipole attractions would be the strongest. However, induced dipole-induced dipole or London dispersion forces would actually be significant because there would be hundreds of such interactions along the chains.

- The most significant interaction with the paper would be hydrogen bonding. The cellulose in the paper would provide hydrogen bond donors (the OH groups) and the side groups in the adhesive chains would provide hydrogen bond acceptors (all those oxygens). However, if the paper contains a lot of sizing, induced dipole-induced dipole or London dispersion forces may form a significant part of the interaction. As with cohesion, this would not be a significant factor except that there are so many alkyl chains involved, so these tiny attractions would add up.



