7.12: Heterogenous Mixtures
- Page ID
- 191993
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)Why does not oil dissolve in water? Oil is composed, in part, of long chains of carbon atoms with hydrogens attached. These chains aren't very polar. It shouldn't be too hard to pull them apart, because they are held together only by London interactions. The chains really aren't long enough to create the strong London interactions that would prevent oil from mixing with water.
On the other hand, it is pretty difficult to pull water molecules away from each other, and the oil does not have the means to do so; it just isn't polar enough. If the water molecules don't move away from each other, there will be no room between them for the individual oil molecules to become dissolved. These two substances will not mix together very well.
Consequently, if placed in the same vessel, they will remain separate and form two different layers. The more dense layer (the water) will sink to the bottom while the lighter, less dense one (the oil) will float to the top.
The same situation is true for a number of other, non-polar organic compounds, such as benzene and toluene. These liquids are too non-polar to dissolve very well in water. Consequently if you mix benzene and water, the two liquids will form two separate layers. Benzene has a specific gravity or density of 0.874 g/mL, whereas the density of water is 1.0 g/mL. As a result, benzene would float on the top, while water would sink to the bttom. Mixing the two layers up as hard as you can may produce temporary mixing (the mixture would form a sort of cloudy, sparkly mess called schlieren), but once left alone the benzene and water would separate out again.
There's one more useful example we should look at. Suppose we have a molecule that is very polar on one end, but non-polar at the other. Soap, for instance, is an ionic compound, but while the cation is usually just a sodium ion, the anion is more complicated. This molecular anion most often contains a very polar "carboxylate group", composed of a carbon with two attached oxygens. It also contains a very long carbon chain, just like in the oil. So one part of the molecule should dissolve well in water, while the other does not. There is a trade-off, and a balance will be struck that determines exactly how soluble the soap is in the water. Interestingly, when placed in water, these soap molecules will arrange themselves in groups so that the polar ends face outward, towards the water, while the nonpolar ends are tucked on the inside. Think of the "circle the wagons" scene in a classic western movie.
There are two reasons why this phenomenon is useful. Micelle formation, as the behavior is called, allows nonpolar substances, such as dirt and oils, to be dissolved in the polar water. The dirt can interact perfectly well with the nonpolar soap tails, and so it will end up in the middle of the micelles, and something that could not be dissolved in plain water turns out to be perfectly soluble in soapy water. But at another level, micelle formation is a very good model for some of the phenomena of cell and molecular biology. For instance, cell membranes are composed of molecules that are somewhat similar to soap molecules. These molecules form groupings similar to large micelles, but with an additional layer of molecules on the inside of the circle, with their polar ends pointing inward. That leaves the nonpolar ends of both layers sandwiched together, out of the water. Proteins also have polar and non-polar regions, and getting the non-polar regions away from the water leads the protein to adopt a specific shape that, in turn, determines the behavior of the protein.
Exercise \(\PageIndex{1}\)

- Answer a:
-
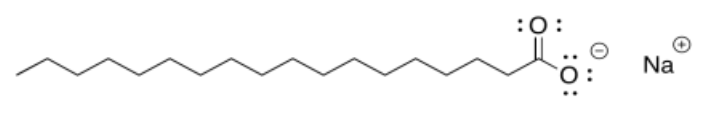
- Answer b:
-
Tail is nonpolar and hydrophobic. Head is polar and hydrophilic.
- Answer c:
-
induced dipole - induced dipole
ion-induced dipole
dipole-induced dipole
ion-dipole
- Answer d:
-
No. The dipole-induced dipole is too weak to overcome hydrogen bonding between waters.
- Answer e:
-
Ion-dipole between water and the hydrophilic head of the soap.
- Answer f:
-
outside
- Answer f:
-
Water is attracted to the hydrophilic heads to form this strong ion-dipole interaction whereas the tails cannot overcome the strong hydrogen bonding of water-water interactions.
- Answer g:
-
Hydrophobic dirt and grease will prefer to interact with the hydrophobic tails (inside micelles).
- Answer h:
-
The grease will be encapsulated in the micelle and washed away in water.


