6.14: Application Problems
- Page ID
- 191305
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)Exercise \(\PageIndex{1}\)
Researchers at Bristol-Myers-Squibb developed a new antidepressant (compound 2, below). The drug binds to GluN2B receptors, shutting down a central nervous system signal that has been linked to depression. Unfortunately, heart problems appeared as a side effect of the medication; the drug also binds to hERG receptors, interfering with potassium ion channels that are important for heart function.
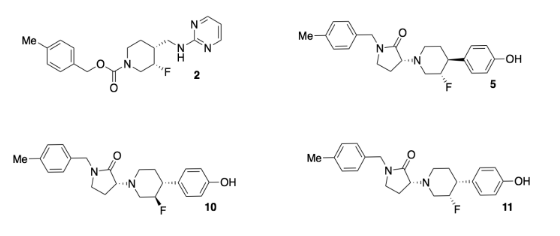
The team designed a new set of compounds (including 5, 10, 11, above. Adapted with permission from Lawrence R. Marcin, Jayakumar Warrier, Srinivasan Thangathirupathy, et al, ACS Med. Chem. Lett. 2018, 9, 472−477. Copyright 2018 American Chemical Society. Used with permission.).
They wanted to make sure the new compounds would still fit tightly in the GluN2B receptor, which is about 9-11 Ångstroms long.
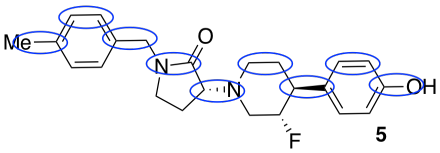
- On compound 5, circle bonds that run roughly parallel to the length of the molecule (i.e. left-right; don’t circle any that are more up-down).
- Count up the number of these in a row. If each one is about 1 Å long, estimate the length of the molecule.
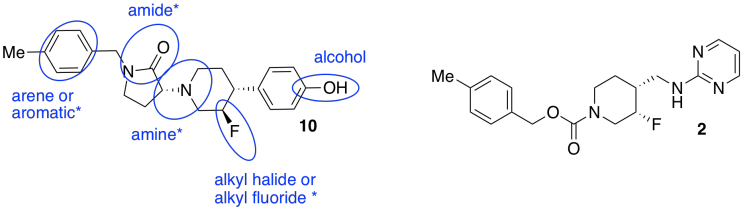
- The functional groups are also important because they control interactions with the receptor. On compound 10, circle and name four of the functional groups. Place an asterisk beside any functional groups found in both compounds 2 and 10.
- What other factor are the researchers clearly looking at between compounds 5, 10, 11? Why would the researchers be interested in that factor?
- State the relationships between compounds 5, 10, 11.
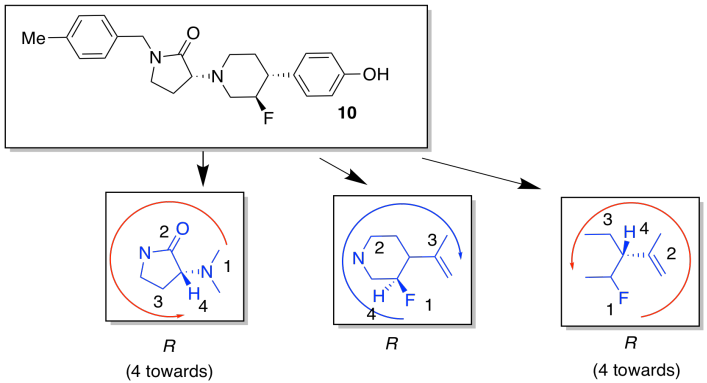
- Find three chiral centers in compound 10. Draw enough of the structure around each chiral center to make it obvious which one you are considering. Rank the priority of the substituents. Assign the configuration (R or S) to each center.
- Researchers added an extra ring in the new compounds. Circle the new ring in compound 10 that has no equivalent in compound 2.
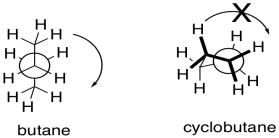
- They incorporated this ring because were interested in adding “conformational rigidity”. To illustrate this idea, draw a Newman projection looking down
i) the middle bond of butane ii) any bond of cyclobutane
Explain why a ring introduces “conformational rigidity” or “conformational restrictions”.
- Why were the researchers interested in adding conformational rigidity?
- Only one of the rings in compound 5 can adopt chair conformations (the others are flat). Draw the two possible chairs. Asses steric strains in each conformer. Indicate which conformer is more stable.
Compound 5 was the most promising candidate; it required the lowest dose for GluN2B but the highest dose to bind hERG.
- Answer a:
-
- Answer b:
-
about 9 Å
- Answer c:
-
- Answer d:
-
Stereochemistry. Because biological receptors are generally proteins containing unique, and chiral, binding sites, they hope to find a stereoisomer that fits the GluN2B receptor, but not the hERG receptor.
- Answer e:
-
These compounds are diastereomers of each other.
- Answer f:
-
- Answer g:
-
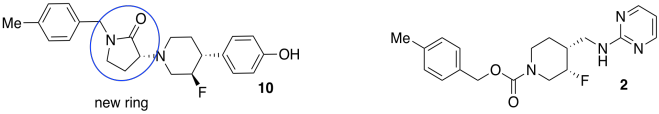
- Answer h:
-
The ring introduces conformational rigidity by preventing the bond from undergoing complete rotation; it is tied back by the connection on the other side of the ring.
- Answer i:
-
They were trying to decrease the number of possible conformations, or shapes, that the compound could adopt. That way, it would be less likely to bind in both the GluN2B receptor and the hERG receptor.
- Answer j:
-
The substituents around the flexible six-membered ring could adopt a number of conformations. We will choose ways that minimize additional steric interactions so that we can focus on the most basic interactions.
Note that amines do not usually have fixed stereochemistry; the fourth substituent on an amine nitrogen is a lone pair, which does not occupy a fixed position in space. Unlike a carbon, the nitrogen can readily switch from one stereochemistry to another.
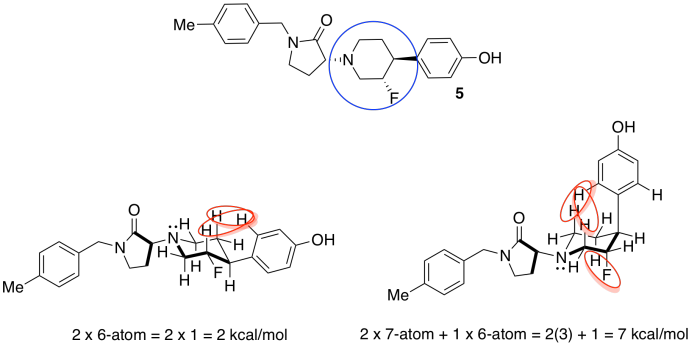
The one on the left, with more equatorial substituents and thus fewer steric interactions, is the more stable one.
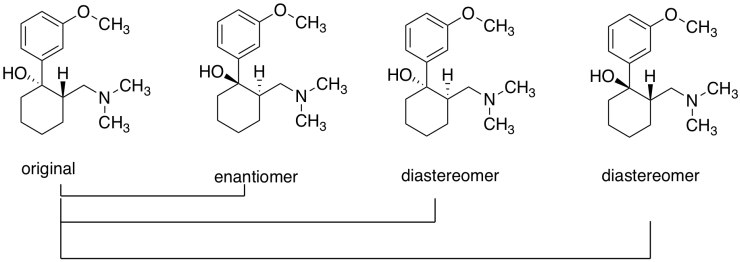
Exercise \(\PageIndex{2}\): Tramadol
Tramadol (below) is an opioid analgesic. It is used to treat chronic pain. It is administered as a racemic mixture; each compound targets a different pain receptor.
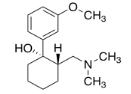
- Draw the enantiomer and any diastereomer(s) of the molecule shown. Label the configurations at the chiral centers.
- If the original molecule is part of the therapeutic tramadol mixture, which other compound is also administered?
- The original compound contains a ring that could adopt different chair conformers. Draw both conformers and assess steric strain in each.
- Which conformer is the more stable one?
- The most stable conformer is not necessarily the medicinally active one. How would dosage need to be adjusted if the least stable conformer were actually the active form?
- Answer a:
-
- Answer b:
-
The original and its enantiomer; the first two above, from the left.
- Answer c:
-

- Answer d:
-
The one on the left, with both groups equatorial and less steric strain, is more stable.
- Answer e:
-
If the one on the left is active, a lower dose can be used because it will spend more time in the active form and be able to bond more receptors. If the less stable one, on the right, is the active form, a higher dose will be needed because only a small fraction of the molecules will be ready to bind the receptor at any given time.


