5.4: Enantiomers
- Page ID
- 189635
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)An atom with four groups attached to it can also adopt a tetrahedral geometry. This geometry often occurs when the central atom is a little smaller. A tetrahedral geometry allows neighbouring groups to get a little farther from each other. The following model of methane shows a pretty simple example. The grey ball is a carbon atom and the four white balls are hydrogen atoms.
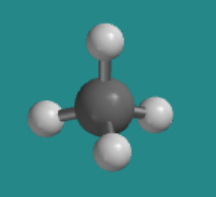
Go to Animation SC3.1. A three-dimensional model of methane, CH4.
Unlike square planar compounds, simple tetrahedral compounds do not have the same kind of cis- and trans- isomers. That is, two groups can't be placed on a tetrahedron so that they are opposite each other or beside each other. The relationship between any two groups on a tetrahedron is the same as the relationship between any other two groups on a tetrahedron.
Dichlorodimethylsilane is a compound that can be used to make silicone polymers. Like platin, it has two each of two groups attached to the central atom. However, the central tom is tetrahedral. There is only one way to arrange these four groups.
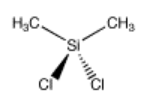
Go to Animation SC3.1. A three-dimensional model of dichlorodimethylsilane.
However, if four different groups are attached to a tetrahedral atom, the four groups can be arranged in two possible ways. The two compounds that result are mirror images of each other. These two isomers are called enantiomers.
For example, suppose that tetrahedral silicon atom had four different groups attached to it: a hydrogen atom, a chlorine atom, a methyl group (that's a carbon with three hydrogens), and a hydroxy group (that's an oxygen with one hydrogen). There would be two slightly different ways to organize those four groups in space. In the picture below, hydrogen is white, chlorine is green, and oxygen is red, whereas silicon and carbon are both grey.
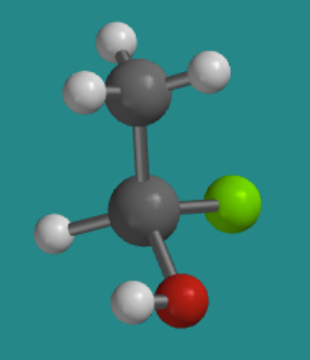
Go to Animation SC3.2. A three-dimensional model of R-chlorohydroxymethylsilane.
Exercise \(\PageIndex{1}\)
Turn the model so that the hydrogen attached to the silicon is hidden behind the molecule. In what direction would you travel if you traced from the chlorine to the oxygen to the carbon: clockwise or counter-clockwise?
- Answer
-
Clockwise
This next picture shows a very similar molecule. The same groups are attached to the central silicon atom, but the are arranged in a slightly different way.
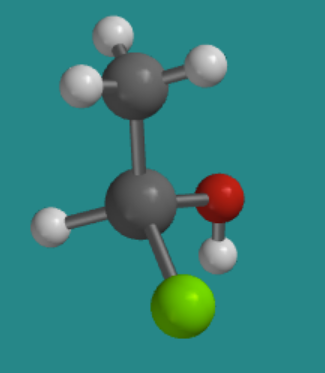
Go to Animation SC3.3. A three-dimensional model of S-chlorohydroxymethylsilane.
Exercise \(\PageIndex{2}\)
Turn the model so that the hydrogen attached to the silicon is hidden behind the molecule. In what direction would you travel if you traced from the chlorine to the oxygen to the carbon: clockwise or counter-clockwise?
- Answer
-
Counter-clockwise
Full disclosure: the molecule we are looking at isn't really a stable compound that you could pour out of a bottle from the chemistry stockroom. That's because one molecule would react with another one just like it, and then another one, and so on to form a long chain called a silicone polymer. That's the kind of material found in things like silly putty and silicone caulking.
Exercise \(\PageIndex{3}\)
Let's try working without the three-dimensional model. The following ball-and-stick picture shows a silicon attached to a bromine (red), a chlorine (green), a fluorine (yellow) and a hydrogen. If you were looking at the molecule with the hydrogen hiddent behind it, in what direction would you travel if you traced from the bromine to the chlorine to the fluorine: clockwise or counter-clockwise?
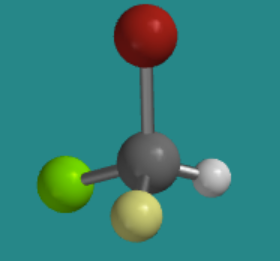
Figure \(\PageIndex{5}\):AnswerCounter-clockwiseBall-and-stick model of an sp3 atom with four different groups attached to it. Bromine is on top, chlorine is to the back left, hydrogen is to the back right, and fluorine points towards the camera.
Exercise \(\PageIndex{4}\)
If you were looking at the molecule with the hydrogen hidden behind it, in what direction would you travel if you traced from the bromine to the chlorine to the fluorine: clockwise or counter-clockwise?
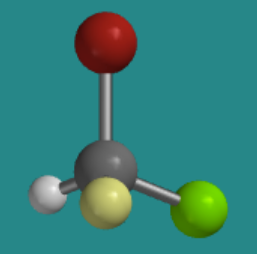
Figure \(\PageIndex{6}\):AnswerClockwiseBall-and-stick model of an sp3 atom with four different groups attached to it. Bromine is on top. Chlorine is to the back right. Hydrogen is to the back left. Fluorine points towards the camera.
There don't have to be four different atoms attached to the central atom to get two stereoisomers. There just have to be four different groups. Even if all four atoms attached to the central one are the same, if they have different things attached to them, then two three-dimensional arrangements are possible. The following compounds illustrate a more subtle example like that. This compound, by the way, is stable and can be isolated and stored; it's a real compound.
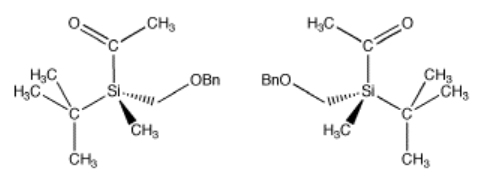
Go to Animation SC2.1. A three-dimensional model of the (-) enantiomer
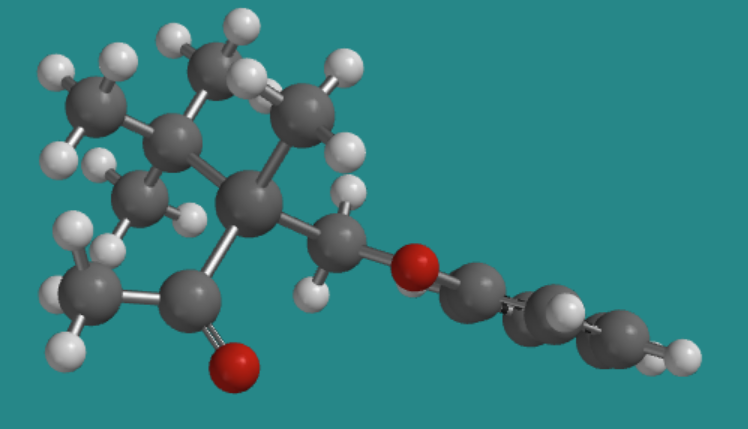
Go to Animation SC2.1. A three-dimensional model of the (+) enantiomer.
- Enantiomers are pairs of compounds with exactly the same connectivity but opposite three-dimensional shapes.
- Enantiomers are not the same as each other; one enantiomer cannot be superimposed on the other.
- Enantiomers are mirror images of each other.
Two compounds with the exact same connectivity, that are mirror images of each other but that are not identical to each other are called enantiomers. The more common definition of an enantiomer is that it is not superimposable on its mirror image. It can be distinguished easily from its mirror image, just as a right hand can easily be identified and distinguished from a left hand.
- Compounds that occur in these pairs are called "chiral".
- "Chiral" comes from the Greek word for "hand".
It can be shown using group theory, the mathematics of symmetry, that an enantiomer may also be defined as a molecule that does not contain a mirror plane, meaning it cannot be divided into two identical and opposite halves.
- Enantiomers contain no mirror planes.
- Enantiomers do not contain two equal and opposite halves.
Unlike cis- and trans-isomers, two enantiomers have the same physical properties. they have the same melting point, the same solubility, and so on. Two compounds that are almost identical, but mirror images of each other, have exactly the same kinds of intermolecular attraction, so it may not be a surprise that their physical properties are identical.
- Enantiomers are another example of a type of stereoisomers.
- Two enantiomers have identical physical properties, except for optical rotation.
Optical rotation involves the interaction of plane-polarized light with a material. If a material is not symmetric, the light that passes through it will be rotated. That means if the waves making up the light are oscillating in one direction as they enter the material, they will have tilted slightly to oscillate in another direction when they emerge from the material. We will look at this phenomenon later.
- Two enantiomers have an equal but opposite rotational effect on plane-polarized light.
- (+) enantiomers rotate light in a clockwise direction.
- (-) enantiomers rotate light in a counterclockwise direction.
For example, in the chiral silicon compound shown above, the (+) enantiomer rotates plane-polarized light in a clockwise direction. It has a "standard optical rotation" of [a] = +12 ( +/-2)o. The (-) enantiomer rotates plane-polarized light in a counterclockwise direction. It has a "standard optical rotation" of [a] = -9.9 ( +/-2)o.
Exercise \(\PageIndex{5}\)
A certain compound exists in two forms; enantiomer A and enantiomer B. Enantiomer A has a molecular weight of 126 g/mol, a density of 0.995 g/mL, an optical rotation of [a] = 26o, a melting point of 65 oC, a boiling point of 225 oC, and an odour of citrus fruit. What can you say about the corresponding properties of enantiomer B?
- Answer
-
Enantiomer B has a molecular weight of 126 g/mol, a density of 0.995 g/mL, an optical rotation of [a] = -26o, a melting point of 65oC and a boiling point of 225oC.
Exercise \(\PageIndex{6}\)
Are these molecules chiral?
Draw the mirror image of each compound and determine if it is superimposable.
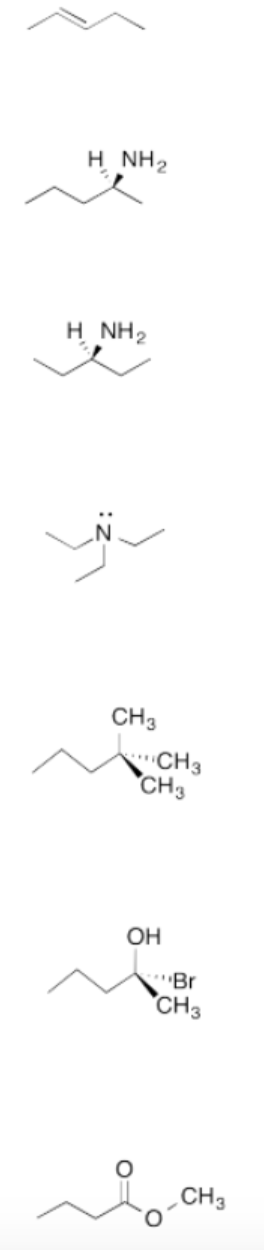
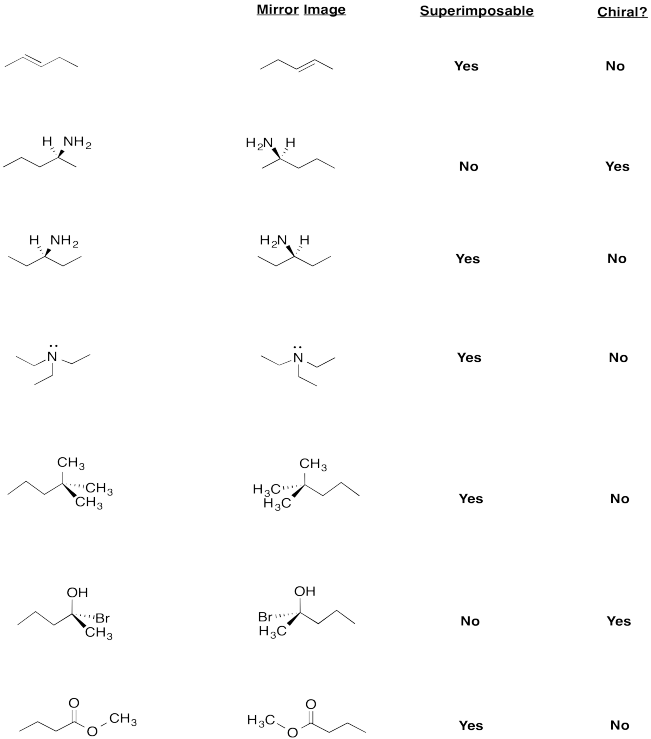
Seven different molecules. From top to bottom: 2-pentene, pentan-2-amine, pentan-3-amine, pentane, 2-bromo-2-pentanol, methyl butanoate.Figure \(\PageIndex{10}\):Answer
Exercise \(\PageIndex{7}\)
Find a mirror plane of symmetry in the following molecules. Some may have no mirror planes and thus are chiral. (Some may have several mirror planes, but it is only necessary to find one in order to determine that the molecule is not chiral.) Hint—there are low barriers to rotation about most single bonds. If any conformer can be drawn in which a molecule has a mirror plane, the molecule will not be chiral.
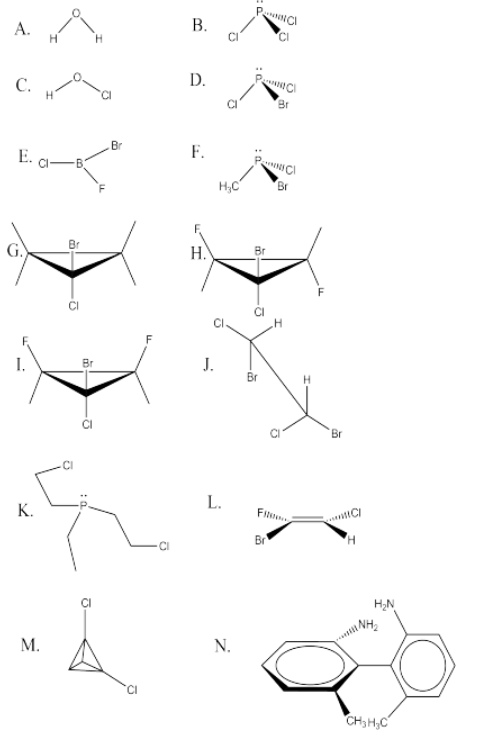
Figure \(\PageIndex{11}\):Answer A.The plane of the page is a mirror plane. There is also one perpendicular to the page that reflects one H into the other.Answer B.The plane of the page contains one P-Cl bond and bisects the other Cl's.Answer C.The plane of the page is a mirror plane.Answer D.Mirror plane contains P-Br bond and bisects the Cl's.Answer E.There is no lone pair on the B. Therefore all atoms lie in a mirror plane.Answer F.No mirror planes--the molecule is therefore chiral.Answer G.There is a plane perpendicular to the page that contains the Br and Cl and bisects the cyclopropane ring.Answer H.No mirror planes--the molecule is therefore chiral.Answer I.There is a plane perpendicular to the page that contains the Br and Cl and bisects the cyclopropane ring.Answer J.The C-C bond can be rotated by 60 degrees so that there is a plane perpendicular to the C-C bond axis.Answer K.The C-C bond in one of the chlorine-containing arms can be rotated so that there is a mirror plane that goes through the ethyl group (with no Cl's) and the P, and one chlorine containing arm is the reflection of the other.Answer L.Since a double bond is planar, there is a mirror plane that contains all six atoms.Answer M.There is a mirror plane that contains two C's and bisects the two Cls.Answer N.No mirror planes--the molecule is therefore chiral. The rings are not in the same plane due to the CH3 and NH2 groups, which bump into each other. They also prevent rotation around the C-C bond between the rings.
Exercise \(\PageIndex{8}\)
Select the picture that illustrates a mirror plane of symmetry in the following compound.
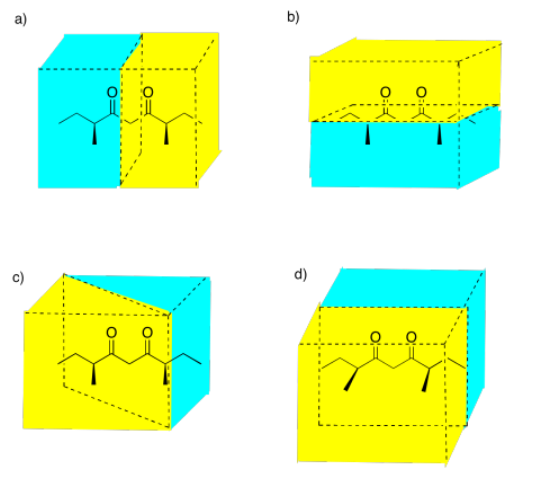
Figure \(\PageIndex{12}\):AnswerPicture (a)Possible answers to Exercise 5.4.8, showing different mirror planes between a molecule of (3S,7S)-3,7-dimethylnonane-4,6-dione
Exercise \(\PageIndex{9}\)
Select the picture that illustrates a mirror plane of symmetry in the following compound.
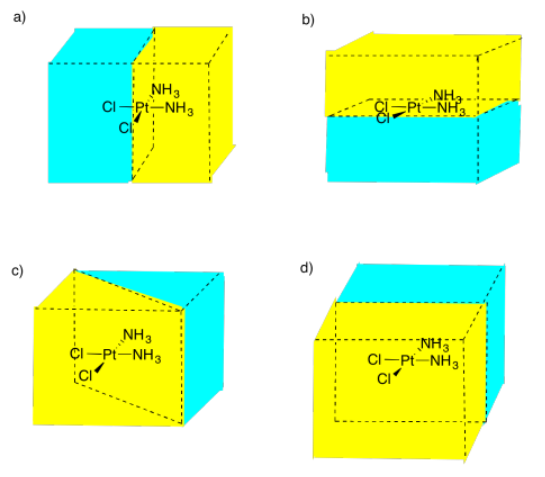
Figure \(\PageIndex{13}\):AnswerPicture (b)Possible answers to Exercise 5.4.9, showing different mirror planes between a molecule of platinum with two chlorine atoms and two ammonia groups.
Exercise \(\PageIndex{10}\)
Select the picture that illustrates a mirror plane of symmetry in the following compound.
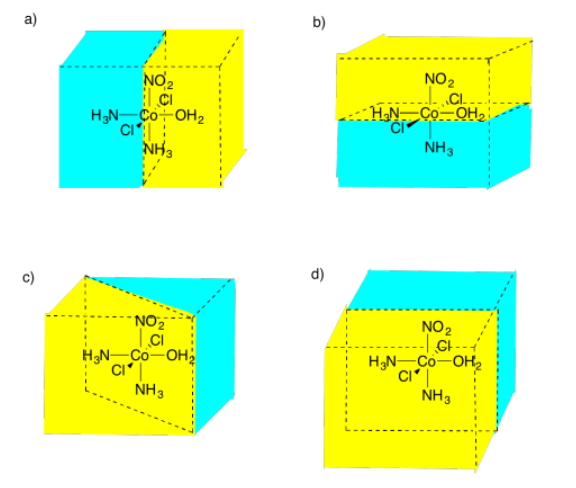
Figure \(\PageIndex{14}\):AnswerPicture (d)Possible answers to Exercise 5.4.10, showing different mirror planes between a molecule of cobalt with two chlorine atoms, two ammonia groups, a nitrate group, and an OH2 group attached.
Exercise \(\PageIndex{11}\)
Select the picture that illustrates a mirror plane of symmetry in the following compound.
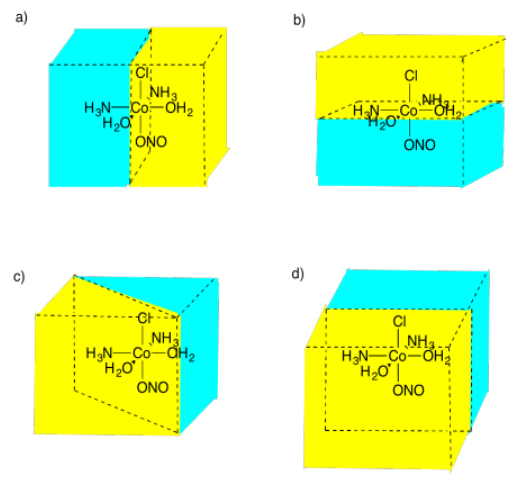
Figure \(\PageIndex{15}\):AnswerPicture (c)Possible answers to Exercise 5.4.11, showing different mirror planes between a molecule of cobalt with a nitrate group, two water groups, two ammonia groups, and a chlorine atom.
Still pictures of models obtained using Spartan 14 from Wavefunction, Inc., Irvine, California.


