3.1: Ionic Atoms
- Page ID
- 189619
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)Periodic trends tell us that some atoms gain electrons easily to obtain a stable configuration; these atoms have a high electron affinity. These atoms are principally found in nature as anions (ANN-eye-ons). Anions are atoms that have gained extra electrons, and have overall negative charge. Examples include the halogens, such as chlorine and bromine.
- Atoms in the upper right section of the periodic table can easily form anions.
One of the most commonly-used periodic properties is electronegativity. A look at the periodic table shows the high electronegativity of atoms in the upper right part of the table. The relatively high positive charge in the nuclei of these atoms makes their attraction for electrons very strong.
Another thing to remember is that atoms towards the right hand side of the periodic table are likely to gain enough electrons to attain the same electron configuration as a noble gas.
The noble gases are exceptional in the periodic table because they do not easily undergo reactions with other elements. In a chemical reaction, electrons are somehow redistributed between atoms. The noble gases have particularly stable electron configurations, however, and do not gain or lose electrons easily. This is true even though they have high electronegativities; in the case of noble gases, high electronegativity might be thought of as a measure of the stability of the electrons in these elements. However, they have a "closed shell" configuration, meaning that any additional electrons would have to go into a much higher energy level in the next "shell". Other atoms can gain a similarly stable electron configuration by gaining or losing electrons. For example, if oxygen gains two electrons, it attains the same electron configuration as neon. Oxygen is frequently found as an O2- anion in many compounds.
Periodic trends also tell us that some atoms lose electrons easily to obtain a stable configuration; these atoms have a low ionization potential. These atoms are principally found in nature as cations (CAT-eye-ons). Cations are atoms that have lost electrons, so that they have an overall positive charge. Examples include the alkalis, such as sodium and potassium.
- Atoms in the vast expanses to the lower left part of the periodic table easily become cations.
Just as some elements attain a noble gas configuration by gaining some extra electrons, other elements can more easily gain that configuration by losing some electrons. For a sodium atom, it is simply closer to get to the same configuration as neon than it is to get to the same configuration as argon. Sodium is frequently found as Na+ in many compounds.
In general, atoms that have an overall charge are called ions (EYE-ons). In an ion, the number of protons in the nucleus is not exactly balanced by the number of electrons, so there is either an overall positive or an overall negative charge on the atom.
What happens to these atoms as they gain or lose an electron? Of course, having an overall charge on an atom introduces an additional electrostatic problem. Two atoms might not repel each other, but two cations would. In the electron sea model of metals, metal cations exist in a block of metal. Those like charges should push the metal cations away from each other, but most metals don't spontaneously fly apart into tiny particles. The cations are partly held together by the presence of delocalized electrons in the block of metal. The attraction of the delocalized electrons for the metal cations offsets any repulsion between the metal cations.
For that reason, we will see that cations are generally found in the presence of anions, and vice versa, so that repulsive forces between like-ions are balanced by attractive forces with oppositely-charged ions.
Given the following pairs of atoms, which one is most likely to give up an electron?
- tantalum or vanadium
- mercury or zinc
- silicon or sulfur
- tungsten or copper
- Answer a:
-
Ta (tantalum) is lower in the periodic table than V (vanadium)
- Answer b:
-
Hg (mercury) is lower in the periodic table than Zn (zinc)
- Answer c:
-
Si (silicon) is to the left in the periodic table compared to S (sulfur)
- Answer d:
-
W (tungsten) is to the left and lower in the periodic table compared to Cu (copper)
Provide the electron configuration for the following atoms or ions (without using the nobel gas abbreviation for the outermost shell). If the atom or ion has the same electron configuration as a noble gas, state which noble gas it resembles.
- oxygen, O
- oxide, O2-
- calcium, Ca
- calcium ion, Ca2+
- aluminum, Al
- aluminum ion, Al3+
- nitrogen, N
- nitride, N3-
- chlorine, Cl
- chloride, Cl-
- Answer a:
-
[He]2s22px22py12pz1
- Answer b:
-
[He]2s22px22py22pz2
- Answer c:
-
[Ar]4s2
- Answer d:
-
[Ar]
- Answer e:
-
[Ne]3s22px1
- Answer f:
-
[Ne]
- Answer g:
-
[He]2s22px12py12pz1
- Answer h:
-
[He]2s22px22py22pz2
- Answer i:
-
[Ne]2s22px22py22pz1
- Answer j:
-
[Ne]2s22px22py22pz2
Notice the change in the name of chloride: frequently, when an element becomes an anion, the end of its name changes to "ide". A chlorine atom that has an extra electron is called a chloride ion. An oxygen atom with two extra electrons (to get to the same electron configuration as neon) is called oxide. A nitrogen atom with three extra electrons (to get to the same electron configuration as neon) is called nitride.
There are other changes that occur when an atom is ionized (when an atom loses or gains an electron, becoming an ion). Cations tend to be relatively small. The higher than normal ratio of positive to negative charge causes the remaining electrons to contract towards the nucleus.
- A cation is always smaller than a neutral atom of the same element.
For similar reasons, anions tend to be relatively large. The low ratio of positive to negative charge causes the remaining electrons to repel each other more and expand away from the nucleus.
- An anion is always much larger than a neutral atom of the same element.
As a result, many of the elements in the periodic table are very different in size depending on whether they form cations or anions.
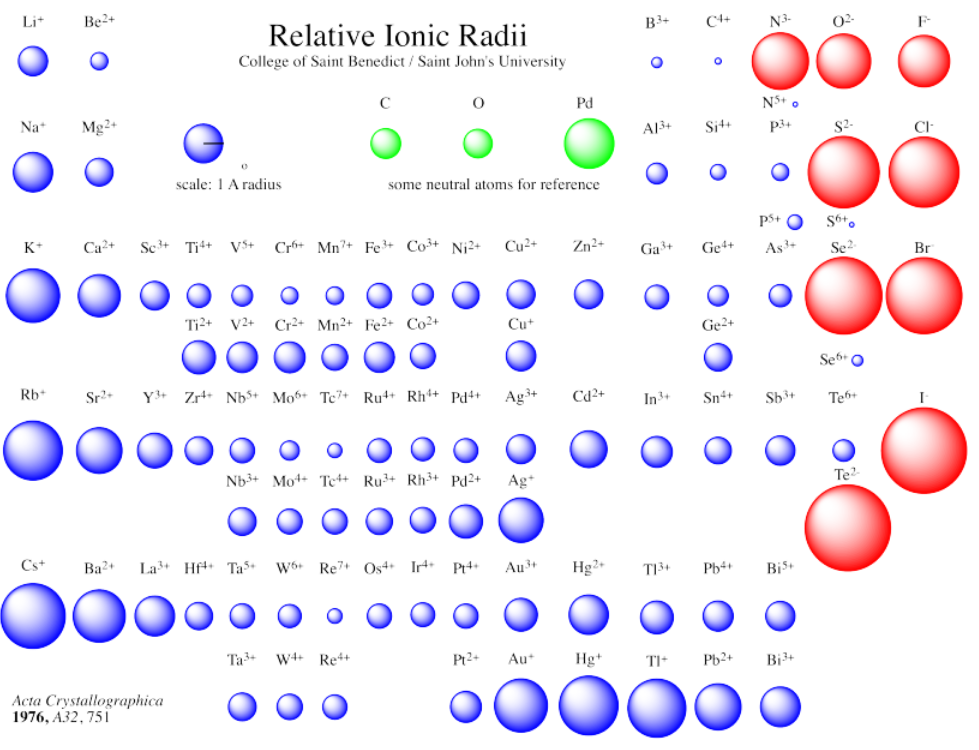
In describing the size of an atom or ion, typically the radius is reported rather than the diameter. Note that different units are sometimes used to report the measured sizes of atoms or ions. The most common unit is an Angstrom, Å. An Angstrom is 10-10 m; it is based on the metric system although it is not, strictly speaking, a metric unit. Alternatively, sometimes atomic and ionic radii are reported in nanometers, nm (10-9 m) or picometers, pm (10-12 m). The reason for the prevalence of the Angstrom is that this unit is about the size of an atom; most atoms have radii between 0.75 and 2 Å. An atom with a 1.0 Å radius has a radius of 100 pm, or 0.10 nm.
Given the following atomic radii, select the correct radius for the given ion.
- Li : 0.134 nm. Li+ : 0.076 or 0.176 nm?
- O : 0.073 nm. O2- : 0.040 or 0.140 nm?
- Cs : 0.235 nm. Cs+ : 0.167 or 0.267 nm?
- H : 0.037 nm. H- : 0.024 or 0.144 nm?
- Answer a:
-
0.076 nm; smaller
- Answer b:
-
0.140 nm; larger
- Answer c:
-
0.167 nm; smaller
- Answer d:
-
0.144; larger
Given the following ionic radii, select the correct radius for the given atom.
- Pb2+ : 0.119 nm. Pb : 0.054 or 0.154 nm?
- F- : 0.133 nm. F : 0.071 or 0.171 nm?
- Pd2+ : 0.086 nm. Pd : 0.057 or 0.128 nm?
- I- : 0.220 nm. I : 0.133 or 0.235 nm?
- Answer a:
-
0.154; larger
- Answer b:
-
0.071; smaller
- Answer c:
-
0.128; larger
- Answer d:
-
0.133; smaller


