Hydrogen Bonding
- Page ID
- 53550
Skills to Develop
- Define and illustrate hydrogen bonds
It's a general rule that "hard" things like to bond with other hard things, and "soft" things like to bond with other soft things. (See the previous section for an explanation of hard and soft.) The reason for this is because hard-hard combinations are very favorable. Remember that hard means "hard to polarize", which usually means small and highly charged. Hard-hard combinations are based on strong Coulomb forces, between relatively big charges relatively close together.
Hydrogen bonds are a special case of hard-hard interaction that occurs in covalent molecules. Hydrogen that is bonded to very electronegative elements (N, O and F) will have a big δ+ (partial positive charge). N, O or F bonded to C or H will have a big δ- (partial negative charge). If the N, O or F has lone pairs, these can make interactions with a hydrogen on another molecule. The partial charges are pretty big, because of the big difference in electronegativity. Also, they can get very close together because these elements (especially H) are very small.
Hydrogen bonds are also an example of a dipole-dipole force, but they are extra big dipole-dipole forces because the charges are big (for partial charges) and the distances are short. They are also an example of Lewis acid-base interactions (because the electrons in the interaction come from a lone pair on N, O or F). And they are also an example of a HOMO/LUMO interaction. The H which is bound to a very electronegative atom has a low LUMO, because the energy match is bad between very different electronegativity atoms, leading to low splitting. The lone pair on the N, O or F is a high HOMO, because it is non-bonding, not bonding. These can then make a new bond.
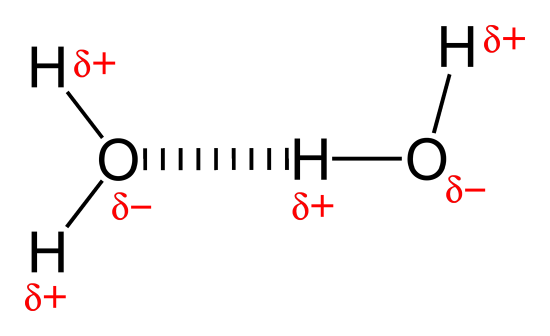
Hydrogen bonds are very important. Because they are very strong, water is a liquid over a much wider temperature range than we would expect otherwise. Hydrogen bonds are also extremely important in biochemistry. They help hold proteins in their correct shape, help DNA store genetic information, and help enzymes make reactions go quickly.
Outside Links
- Khan Academy: Van der Waals Forces (12 min)
- CrashCourse Chemistry: Liquids (11 min)
Contributors and Attributions
Emily V Eames (City College of San Francisco)

