Ancient History
- Page ID
- 54095
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\dsum}{\displaystyle\sum\limits} \)
\( \newcommand{\dint}{\displaystyle\int\limits} \)
\( \newcommand{\dlim}{\displaystyle\lim\limits} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\(\newcommand{\longvect}{\overrightarrow}\)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)Skills to Develop
- Distinguish physical changes from chemical changes
- Identify the three states of matter and their respective properties
- Understand some of the history behind identifying the three states of matter
- Define matter and its components (i.e. mass, weight, volume)
Humanity's first chemical knowledge was mostly technology, like metal working, ceramics, cooking, etc. Early civilizations learned to control fire, to cast metals and make alloys, to make glass and ceramics, and so forth. The first chemical thinking, as opposed to chemical applications, asked: What is matter? Matter is stuff. It's what we are made of, what the earth and the air are made of. Matter is anything that has mass... what is mass? It's the amount of stuff. Not how much space it occupies (that's volume) but how much stuff is there. We measure mass using weight, which is how strongly the stuff in question is attracted to the earth by gravity.
What is matter made of? Well, one philosopher of ancient Greece proposed that all matter is made of water. He observed that water can "become air" by evaporation, or become solid by freezing into ice. He reasoned therefore that water can convert into everything, and matter is made of water. Now, we call those changes physical changes. The water is still water when it boils and turns into steam. The water is still water when it freezes into ice. We changed its temperature, not its nature.
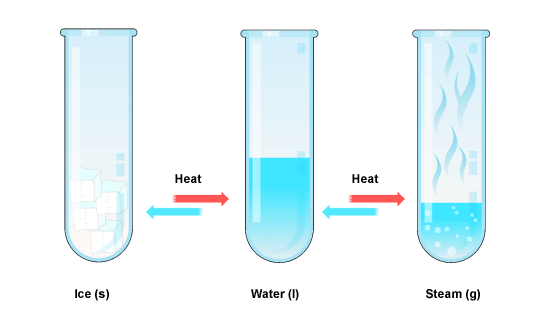
Another Greek philosopher said that everything was made of air: when air becomes less compressed, it becomes fire, and when more compressed, it turns into water, stones, and so forth. He offered the proof that when you breathe through open lips, the air is warm, and when you compress it by breathing through puckered lips, it's cold, and condenses into liquid or solid. Air turning into stone would be a chemical change, in modern terms. In terms of Dalton's Atomic Theory, a chemical change means that the atoms form new combinations, like one atom of A combining with an atom of B. Before A and B were separate, but now they are attached. That's a chemical change.
Others proposed 5 elements, with distinct shapes: octahedra, tetrahedra, cubes, etc. For a long while, the four element model (earth, air, fire, water) was popular. (In Greece it was proposed by a man who was asked to become king of his city, but created a democracy instead. Then he declared himself a god and jumped into a volcano. It's said that the volcano tossed back his sandals to prove he wasn't a god.) This model, which Plato and Aristotle also used, suggested that all matter was composed of these four elements in different ratios. For instance, when wood is burned, you get smoke (air), ash (earth), pitch (a viscous liquid, here identified with water), and fire, so wood is made of all these things. Wood burning is a great example of a chemical change.
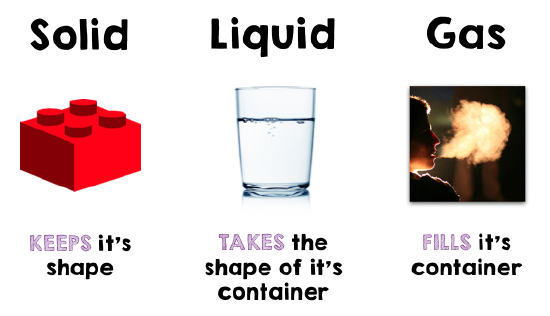
From a modern perspective, in all these theories an important element was lacking: experimentation. The Greeks preferred thinking to trying things, and you might say that it shows in their theories. For them, the fundamental difference was what we now call the state of matter or the phase of matter. Solid, liquid and gas are the three main states of matter.
- Ice, wood and stone are all solids
- Water is a liquid, like oil
- Air is a gas, like steam, and like the gas that you use in a stove
The three states are fundamentally different in nature. Gases take up as much space as they can, in whatever shape they can: the molecules are far apart, and try to spread out. Liquids change shape but have a constant volume. Solids have a constant shape and volume. Now we know that you can change the state of matter just by changing the temperature and pressure, and the molecules stay the same. So the wrong theories of the Greeks were based on not recognizing the difference between physical changes, which are changes in the state of matter, and chemical changes, changes in the combination of atoms.
Summary
Matter is anything that has volume and mass. Mass is the amount of matter in a substance. Physical changes involve processes that change the form of a substance but not its chemical composition. Chemical changes involves processes in which a new substances are formed with new chemical compositions. Weight is the force resulting from a mass being pulled towards a particular location as a result of gravity. The three states of matter are: solid, liquid, and gas. A solid is an object with a fixed shape and volume. Liquids are fluids have a constant volume but are capable of changing their shape. Gases are fluids that do not have constant shape or volume so they take up as much space as they can within a given container.
Contributors and Attributions
Emily V Eames (City College of San Francisco)

