2.1: Introduction to Biomolecules and Cell Components
- Page ID
- 308327
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\dsum}{\displaystyle\sum\limits} \)
\( \newcommand{\dint}{\displaystyle\int\limits} \)
\( \newcommand{\dlim}{\displaystyle\lim\limits} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\(\newcommand{\longvect}{\overrightarrow}\)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)- 1: Define the basic structure of biomolecules, such as: amino acids and proteins, carbohydrates, fatty acids, triacylglycerol, phospholipids, steroids and nucleic acids.
- 2: Define the meaning and significance of essential and non-essential amino acids.
- 3: Understand the function of enzymes.
- 4: Define the basic structure of ribonucleic acid (RNA) and deoxyribonucleic acid (DNA).
1.1
Amino Acids and Proteins
Amino acids are the basic units of proteins. All amino acids present in proteins carry a carboxyl- and an amino group, hydrogen and variable side chains (R) at a single α – carbon atom.
Amino Acid Basic Structure:
Every amino acid has four components linked together with a central carbon atom α – carbon:
- Amino group (NH2)
- Carboxylic acid group (COOH)
- Hydrogen atom (H)
- R-group, which varies with each amino acid (R)
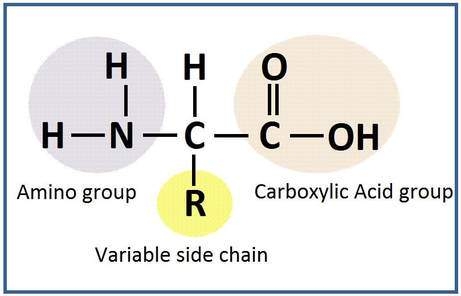
R groups may be:
- Hydrophobic
- Hydrophilic
- Charged R-groups: positive or negative charged
- Special R-groups: conjugated with other molecules
Amino Acids are classified into two groups.

Essential: Humans cannot synthesize them and must be obtained directly from food (phenylalanine, valine, threonine, tryptophan, methionine, leucine, isoleucine, lysine, histidine, cysteine, and arginine).
Non-essential: The human body is able to produce them (glycine, alanine, serine, asparagine, glutanine, tyrosine, aspartic acid, glutanic acid, and proline).
Below, you can see different structures of the most common amino acids in humans. Amino acids link together, in a reaction known as peptide bond, to form proteins.


Levels of Protein Structure
Primary (1°) Structure
The sequence of amino acids in a protein is named as primary structure. The amino acids are linked via peptide bonds formed with the carboxylic acid group of one amino acid and the amino group of a other amino acid.
Secondary (2°) Structure
The secondary structure is the way a polypeptide folds to form α-helix, β-strand, or β-turn.
Tertiary (3°) Structure
The tertiary structure is the way the polypeptide chain coils and turns to form a complex molecular shape. Additionally, tertiary structure starts to develop an active sites of proteins where critical actions and interactions will take place.
Quaternary (4°) Structure
The quaternary structure is the combination of the multiple protein subunits that interact to form a single, larger, biologically active protein.
Protein Functions
Proteins have several functions in the human body including hormonal, enzymes, structural proteins in cell membranes, proteins also receive signals from outside the cell and mobilize intracellular response, and they are part of the immune system.
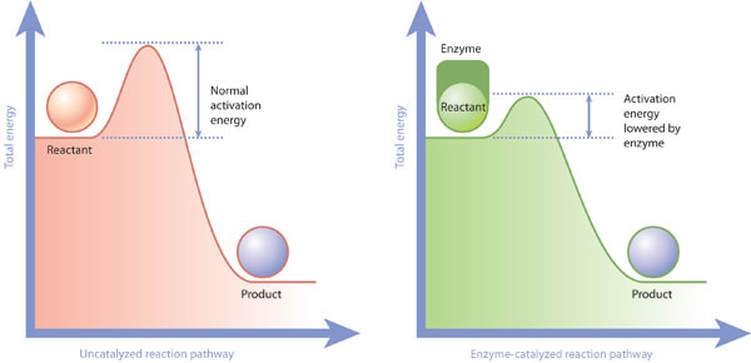
Enzymes are specialized proteins that accelerate a chemical reaction by serving as a biological catalyst. By catalyzing these reactions, enzymes cause them to take place one million or more times faster than in their absence. Several biochemical reactions important for cellular maintenance occur due to enzymes activity. For example: environmental response and metabolic pathways.
1.2
Carbohydrates
 Carbohydrates are made of molecules of carbon (C), Hydrogen (H) and Oxygen (O), and are composed of recurring monomers called monosaccharides (which typically form ring structures). A common name of monomers and dimers is ‘sugar’.
Carbohydrates are made of molecules of carbon (C), Hydrogen (H) and Oxygen (O), and are composed of recurring monomers called monosaccharides (which typically form ring structures). A common name of monomers and dimers is ‘sugar’.
Carbohydrates are classified into three subtypes.

1. Monosaccharides: 1 unit of monomer. Examples: fructose, glucose, galactose.
Present in fruits, etc.
2. Disaccharides: 2 units of monosaccharides. Examples: lactose, maltose, sucrose.
Present in milk, etc.
3. Polysaccharides: Many monosaccharide units. Examples: cellulose, glycogen, starch.
Present in breads, grass, etc.
Carbohydrates are a group of macromolecules that are important energy source required for various metabolic activities. Carbohydrates may bind to proteins and lipids that play important roles in cell interactions e.g. receptor molecules and immune system e.g. antigens.

1.3
Lipids
Lipid molecules are mainly hydrophobic molecules i.e. found in areas away from water molecules, but also present smaller hydrophilic parts that are important for its biological function. The major roles of lipid molecules are to serve as storage of biological energy (Example: triacylglycerols) and provide the building blocks for biological membranes (Example: phospholipids and cholesterol). Although there are other types of lipids, in this topic we will discuss the structure and function of these main groups of lipids.
Triacylglycerols
Triacylglycerols are composed of fatty acids and glycerol.
- Glycerol is a simple three-carbon molecule with hydroxyl groups at each carbon.
- Fatty acids are chains of carbon molecules with a carboxylic acid (COOH) in the first carbon and a CH3 (methyl) group at the end of the chain.

Fatty acids can be...
Saturated: Fatty acids contain only single carbon-carbon bonds, and all of the carbon molecules are bonded to the maximum number of hydrogen molecules.
Unsaturated: Fatty acids have at least one double carbon-carbon bond with the potential for additional hydrogen atom bonding still existing for some of the carbon atoms in the backbone chain. If more than one double bond is present, the term polyunsaturated is used.
Essential Fatty Acids
Examples of two essential fatty acids, linoleic acid (known as omega-6;ω-6) and linolenic acid (known as omega-3; ω-3). These fatty acids present double bonds at the sixth and third carbon atoms, respectively, counting from the methyl end of their chains. They are considered essential because humans do not have the ability to produce double bonds at these locations and, therefore, must obtain these two fatty acid from vegetable oils.

Phospholipids
Phospholipids are the major component of cell membranes. They form lipid bilayers because of their amphiphilic characteristic.
The structure of the phospholipid molecule generally consists of two hydrophobic fatty acid "tails" and a hydrophilic "head" consisting of a phosphate group (PO4−3) attached to the third glycerol carbon. This head group is usually charged, creating a part of the lipid that is hydrophilic, and wants to be near water, a quality that is essential for the formation of biological membranes and many lipid functions.

Steroids
Steroids are lipids that have four rings made of carbon atoms—three rings have six sides and one has five sides—with a six-carbon ring tail. Examples: bile salts, cholesterol, the sexual hormones estrogen, progesterone and testosterone, corticosteroids and pro-vitamin D.
Cholesterol
Cholesterol is an important molecule found only in eukaryotic organisms with a variety of functions. Cholesterol is also a component of biological membranes and its main function is to control the fluidity of membranes. Cholesterol does not like to be exposed to water environments, preferring to be shielded by other hydrophobic molecules such as lipids or hydrophobic parts of proteins.
Cholesterol also serves as the primary source for the production of steroid hormones, bile salts, and vitamin D.

1.4
Nucleosides and nucleotides are involved in the preservation and transmission of the genetic information of all living creatures. In addition, they play roles in biological energy storage and transmission, signaling and regulation of various aspects of metabolism.
These molecules can be divided into two major families.
 Purines: They are two-ring structures: adenine and guanine.
Purines: They are two-ring structures: adenine and guanine.
Pyrimidines: They are one-ring structures: thymine, cytosine, and uracil.
The unique structure and interaction of these molecules serve as the fundamental building block of RNA and DNA molecules and allow fundamental processes of DNA replication and protein synthesis to occur.
Components of Nucleotides
- Nitrogenous base: The nitrogenous base of a nucleoside or nucleotide may be either a purine or a pyrimidine.
- Carbohydrate: The carbohydrate component of nucleosides and nucleotides is usually the sugar ribose for RNA molecules and deoxyribose for DNA molecule
- Phosphate Group: One or more phosphate groups (PO4−3) may be attached to the carbon 5 of the carbohydrate molecule.

1.5
DNA
DNA stands for deoxyribonucleic acid.
It is an extremely long molecule that forms a double-helix.

DNA components:
- Sugars - Deoxyribose
- Phosphates - (PO4−3)
- Base - cytosine (C), guanine (G), adenine (A) and thymine (T).
The DNA consists of two strands attached to each other by hydrogen bond created by nucleotide pairing (A-T and C-G).
The double-helix structure of DNA is important for its function because these two bonded strands can temporarily separate to allow for DNA replication.
The sequences of nucleotides (A, C, T, G) in the DNA molecule will make up the genes and, subsequently, proteins are referred to as “expressed sequences” or “exons.” Sequences that do not code for a protein are called “intervening sequences” or “introns.”

Human Genome
The genome of humans is estimated to contain approximately 20,000–25,000 different genes arranged on multiple chromosomes.
Twenty three pairs of chromosomes:
Twenty two pairs (autosomes).
One pair (sex chromosome) (xx) (female) or (xy) (male).
Humans have 23 pairs of chromosome in every cell (except mature red blood cells); Gametes or sex cells (sperm and eggs) have half the normal complement of chromosomes.
1.6
RNA 
RNA stands for ribonucleic acid.
RNA molecules are single strands.
RNA components:
- Sugars - Ribose
- Phosphates - (PO4−3)
- Base: cytosine (C), guanine (G), adenine (A) and uracil (U)
RNA molecules often form secondary (2°) structures and may interact with DNA, other RNA molecules, and proteins. These interactions help to define the particular function of each type of RNA.
Types of RNA molecules and functions:
Messenger RNA (mRNA):
Molecules which function as the transmitter of genetic information from the DNA genetic code to the resulting protein.
Transfer RNA (tRNA)
Molecules that carry amino acids and match them with a specific mRNA sequence during protein synthesis.
Ribosomal RNA (rRNA)
Molecules associated with proteins and are responsible for the synthesis of protein molecules.
Regulatory RNA
Molecules involved in regulation of DNA expression, posttranscriptional mRNA processing, and the activity of the transcribed mRNA message.
The basic structure of DNA and RNA are similar, however with 3 main differences:
 Nitrogenous Bases: Three of the nitrogenous bases are the same in the DNA and RNA: adenine, cytosine, and guanine. The fourth base for DNA is thymine while for RNA it is uracil. Thymine and uracil both bind to adenine.
Nitrogenous Bases: Three of the nitrogenous bases are the same in the DNA and RNA: adenine, cytosine, and guanine. The fourth base for DNA is thymine while for RNA it is uracil. Thymine and uracil both bind to adenine.
Number of Strands: The DNA molecule is usually double-stranded and most cellular RNA molecules are single-stranded.
Type of Sugar: In the DNA molecule the sugar is deoxyribose and in the RNA molecule the sugar is ribose.
Topic 1: Key Points
In this section, we explored the following main points:
- 1: Amino acids link together, in a reaction known as peptide bond, to form proteins.
- 2: One important function of protein is to act as enzymes to accelerate chemical reactions.
- 3: Carbohydrates are important energy source required for various metabolic activities and may bind to proteins and lipids that play important roles in cell interactions
- 4: Lipid molecules serve as storage of biological energy and provide the building blocks for biological membranes
- 5: DNA and RNA structures have 3 main differences .The nitrogenous bases (DNA has thymine and RNA has uracil). The DNA molecule is usually double stranded and most of the RNA molecules are single stranded. In the DNA molecule the sugar is deoxyribose and in the RNA molecule the sugar is ribose.
1. What type of nucleic acid does thymine belong to?
- Answer
-
DNA
2. Uracil?
- Answer
-
RNA
3. Enzymes are...
specialized proteins that accelerate a chemical reaction by serving as a biological catalyst.
specialized proteins that stops a chemical reaction.
- Answer
-
specialized proteins that accelerate a chemical reaction by serving as a biological catalyst.
4. A nucleotide consists of...
Check all that apply.
- A sugar (either deoxyribose or ribose )
- Uracil as the nitrogen base
- A phosphate group
- One of the four nitrogen bases
- Answer
-
A sugar, A phosphate group, and One of the four nitrogen bases
5. Lipid molecules are mainly hydrophilic molecules. True or False?
- Answer
-
false


