15.10: Nuclear Energy
- Page ID
- 285729
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)Nuclear energy is generated by the neutron-induced fissioning of heavy atomic nuclei, most commonly those of the uranium isotope with a mass number of 235 or plutonium with a mass number of 239, to produce radioactive fission products, an average of 2.5 more neutrons and an astounding amount of energy compared to an ordinary chemical reaction. A typical example of such a fission reaction is the following:
\[\ce{^{235}_{92}U + ^{1}_{0}n \rightarrow ^{133}_{51}Sb + ^{99}_{41}Nb + 4 ^{1}_{0}n}\]
A nuclear reactor operating at a constant power level is controlled such that on average 1 neutron from each fission reaction is absorbed to cause another fission reaction, thus sustaining a chain reaction. The excess neutrons are absorbed by nonfissionable material. In order to cause the desired fission, the neutrons, initially released as rapidly moving, high energy particles must be slowed down, which is done by a moderator, such as water, in the reactor.
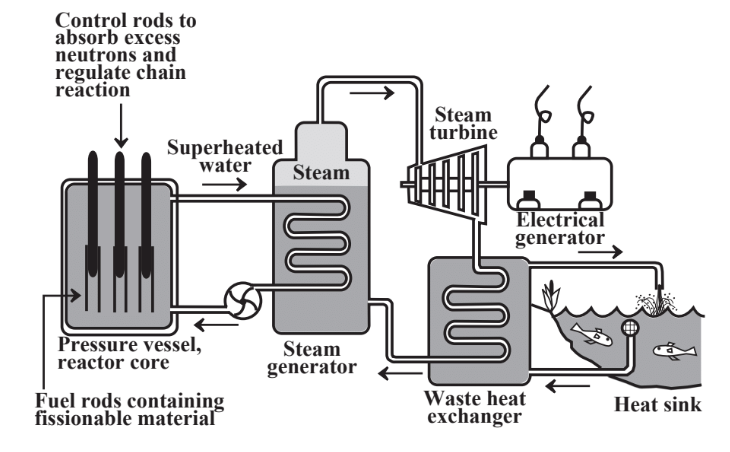
The basic function of a nuclear power reactor is to serve as a heat source to produce steam used to generate mechanical energy. The basic components of a nuclear power reactor are shown in Figure 15.13. Pressurized superheated water circulates through the hot reactor core in an enclosed loop (to prevent escape of radioactive contaminants). Heat from this water is used to convert water to steam in a heat exchanger. The rest of the power plant is like a conventional fossil-fueled plant with a steam turbine coupled to a generator and the steam from the steam turbine being condensed to provide liquid water for the heat exchanger.
Although only 0.71% of natural uranium is fissionable uranium-235, and uranium to be used for fission must be enriched in this isotope, there is an adequate global supply of uranium. In principle, the remaining 99.28% of uranium that consists of uranium-238 could be converted to fissionable plutonium by absorption of neutrons in breeder reactors. Plutonium is actually generated by uranium-238 absorbing neutrons in a conventional nuclear power reactor, and after the reactor has operated for a few months after refueling, a large fraction of its energy output comes from plutonium generated in the reactor.
An interesting possibility for breeder reactors is the liquid-sodium-cooled travelling wave reactor in which a relatively small segment at the end of a mass of uranium held in an array of fuel rods is enriched in fissionable uranium-235. The fission process is initiated in this portion of the fuel and neutrons from it migrate to the adjacent non-enriched segment where they are absorbed by uranium-238 to produce fissionable plutonium-239. This builds up a sector containing enough plutonium to sustain fission and the nuclear fission process very slowly and continuously migrates into the area with the newly fissionable material. With proper design such a reactor can function for decades before the “wave” reaches the end of the mass of uranium and no more fissionable material is produced to sustain fission.
A major issue with nuclear power reactors is posed by the high-level nuclear wastes consisting of radioactive fission products generated when the uranium nucleus splits apart and the radioactive transuranic elements produced when uranium nuclei absorb neutrons. Some of the radioactive fission products may last for several centuries and some of the transuranic isotopes remain lethal for thousands of years. At the present time, spent fuel elements are being stored under water at the reactor sites. This is actually a good thing because the short-lived fission products that are responsible for most of the radioactivity in nuclear fuel freshly removed from a reactor decay rapidly, and after a few years of storage only a small fraction of the original activity is present. Under current regulations in most countries, the wastes from this fuel will eventually have to be buried. A better alternative is to process the material in the spent fuel elements to remove radioactive products from uranium fuel. The relatively short-lived fission products decay spontaneously within several hundred years and can be stored in a secure location for several centuries. The longer-lived nuclear transuranic wastes can be bombarded with neutrons in nuclear reactors, a process of transmutation in which the elements are converted to other elements or fission products with shorter half lives resulting in relatively rapid production of stable isotopes. Radioactive waste elements for which transmutation is feasible include plutonium, americium, neptunium, curium, technetium-99, and iodine-129. Plutonium, americium, neptunium, and curium are heavy actinide elements that are fissionable and add fuel value in a nuclear reactor.
Another problem with nuclear reactors is their decommissioning. One option is to dismantle the reactor soon after it is shut down using apparatus operated by remote control. The radioactive reactor parts are then disposed. Another approach is to allow the reactor to stand for 30–100 years before dismantling, by which time most of the radioactivity has decayed (and most of the people responsible for the reactor initially have died). A third option is to entomb the reactor in a concrete structure.
Two accidents have dealt a strong blow to the future of nuclear energy. The first, and much lesser of these, occurred on March 28, 1979, when Metropolitan Edison Company’s nuclear reactor located on Three Mile Island in the Susquehanna River, 28 miles outside of Harrisburg, Pennsylvania, lost much of its coolant resulting in overheating, and partial disintegration of the reactor core. Some radioactive xenon and krypton gases were released to the atmosphere and some radioactive water entered the river. The problem was remediated and the reactor building sealed. Then in April of 1986 a reactor of inherently dangerous Soviet design blew up in Chernobyl, which is now part of Ukraine. Officially, 31 people were killed, but the death toll was probably many more, especially when delayed effects of exposure to radioactive materials are considered. Food, including reindeer meat in Lapland, was contaminated as far away as Scandinavia, thousands of people were evacuated, and the entire reactor building was entombed in a massive concrete structure. The reactor that blew up was one of four units, the last of which was not shut down permanently until the end of 2000!
Given the horrors described above, why would reputable scientists even advocate development of nuclear energy? The answer is, simply, carbon dioxide. With massive world resources of coal and other non petroleum fossil fuels, the world has at least enough readily available fossil fuel to last for a century. But evidence is mounting that the carbon dioxide from fossil fuel combustion is leading to global warming accompanied by effects such as rising sea levels that will inundate many coastal cities. Humans do know how to design and operate nuclear reactors safely and reliably; indeed, France has done so for years and gets most of its electricity from nuclear fission, and the U.S. Navy has had an exemplary safety record with reactors on submarines and aircraft carriers. So, it may be that nuclear energy is far from dead and that humankind, reluctantly and with great care, will have to rely on it as the major source of energy in the future. A new generation of nuclear power plants is waiting to be built that have the desirable characteristics of passive stability. This means that measures such as gravity feeding of coolant, evaporation of water, or convection flow of fluids operating automatically provide for safe operation of the reactor and automatic shutdown of the reactor if something goes wrong. New designs are also much more reliable with only about half as many pumps, pipes, and heat exchangers as are contained in older power reactors.
Nuclear Fusion
The fusion of a deuterium nucleus and a tritium nucleus releases a lot of energy as shown below, where Mev stands for million electron volts, a unit of energy:
\[\ce{ ^{2}_{1}H + ^{3}_{1}H \rightarrow ^{4}_{2}He + ^{1}_{0}n + 17.6 MeV} \, \text{(energy released per fusion)}\]
This reaction is responsible for the enormous explosive power of the “hydrogen bomb.” So far it has eluded efforts at containment for a practical continuous source of energy. And since physicists have been trying to make it work on a practical basis for the last approximately 60 years, it will probably never be done. (Within about 15 years after the discovery of the phenomenon of nuclear fission, it was being used in a power reactor to propel a nuclear submarine.) However, the tantalizing possibility of using the essentially limitless supply of deuterium, an isotope of hydrogen, from Earth’s oceans for nuclear fusion still give some investigators hope of a practical nuclear fusion reactor.
Nuclear fusion was the subject of one of the greatest scientific embarrassments of modern times when investigators at the University of Utah in 1989 announced that they had accomplished so-called cold fusion of deuterium during the electrolysis of deuterium oxide (heavy water). The announced “discovery” of cold fusion resulted in an astonishing flurry of activity as scientists throughout the world sought to repeat the results, whereas others ridiculed the idea. Unfortunately, for the attainment of a cheap and abundant source of energy, the skeptics were right, and the whole story of cold fusion stands as a lesson in the (temporary) triumph of wishful technological thinking over scientific good sense.


