15.9: Carbon Sequestration for Fossil Fuel Utilization
- Page ID
- 285727
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)Fossil fuels can be used sustainably by employing carbon sequestration to retain greenhouse gas carbon dioxide produced in fuel combustion or coal conversion, which by preventing carbon dioxide generated by fossil fuels from entering the atmosphere holds the promise of enabling utilization of fossil fuels without contributing to greenhouse warming. Carbon dioxide can be captured and sequestered deep in the ocean or pumped into porous formations underground. A major concern with oceanic disposal of carbon dioxide is the tendency of this gas to lower pH resulting in thinner shells in shellfish.
The most promising approach to carbon sequestration is to pump carbon dioxide gas into underground formations at depths exceeding 1000 meters. If the formations are overlain with impermeable layers of rock that are not breached by improperly abandoned oil wells, the carbon dioxide will remain in place indefinitely. Sequestration is aided by the presence of saline groundwater and by chemical reaction with minerals.
Carbon dioxide from natural gas that contains a high content of CO2 has been sequestered since 1996 in the Sleipner oil and gas field about 240 km off the Norwegian coast. The gas is pumped into the 200-m thick Utsira sandstone formation located about 1000 km below the seabed. A mixture of carbon dioxide and toxic hydrogen sulfide is now being disposed underground in Alberta, Canada.
Carbon dioxide is best captured and sequestered in processes such as fermentation of sugars to make ethanol that produce the gas in high concentrations. Because of the high nitrogen contents exhaust gases from combustion are not suitable for carbon dioxide sequestration. Using pure oxygen instead of air for combustion does produce a relatively pure carbon dioxide product and one commercial power plant using coal burned in pure oxygen is now planned with support of the U.S. Department of Energy.
The most promising approach for large-scale carbon dioxide sequestration is through coal gasification (see Section 15.8). There are two major sources of carbon dioxide from coal gasification. The first of these is coal combustion with pure oxygen oxidant,
\[\ce{C(coal) + O2 \rightarrow CO2 + heat}\]
which generates the heat required for gasification of the hot carbon residue of coal with steam:
\[\ce{C(coal) + H2O \rightarrow CO + H2}\]
The second reaction that produces carbon dioxide is reaction of steam with CO to increase the ratio of H2 to CO in the synthesis gas product:
\[\ce{CO + H2O \rightarrow H2 + CO2}\]
The largest carbon dioxide sequestration process now operating in the U.S. is the Great Plains Synfuels Plant near Beulah, North Dakota. This plant gasifies 16,000 tons per day of lignite coal and sends approximately 4.3 million cubic meters of carbon dioxide per day (3 million tons per year) through a 330 km pipeline to the Weyburn and Midale oilfields in Saskatchewan, Canada, for sequestration and petroleum recovery.
An integrated coal gasification plant with carbon dioxide sequestration is shown in Figure 15.12. This plant uses the reaction of steam with hot carbon from coal to produce elemental hydrogen (reaction 15.9.2) and reacts the CO product with steam to produce more H2; both of
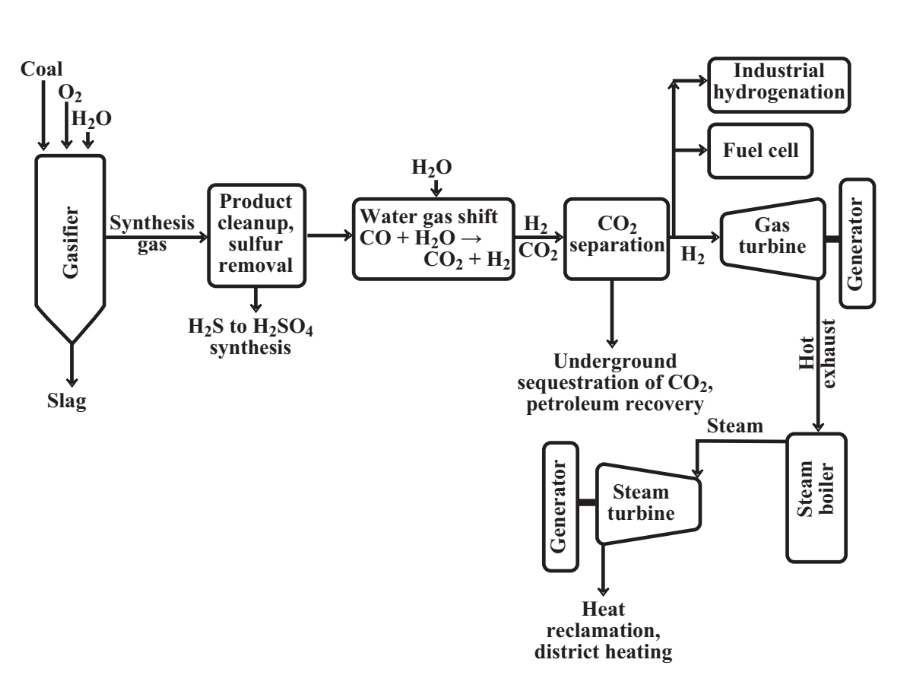
these reactions produce CO2. A gas turbine fueled with H2 coupled to a generator produces electricity. Hot exhaust gas from the gas turbine is used to raise steam in a boiler and this steam powers a steam turbine that is also coupled to a generator. This combination results in very efficient electrical power generation. The elemental H2 generated can be used in fuel cells, as a chemical reagent to produce synthetic hydrocarbon fuels, or to synthesize ammonia, NH3. Some ammonia is also produced from nitrogen in the coal during gasification and is recovered as a product. Sulfur, which occurs in essentially all coals, is released during gasification as byproduct hydrogen sulfide used to make sulfuric acid or disposed with the carbon dioxide byproduct. The CO2 is separated from the gas and pumped under high pressure into mineral formations at depths up to around 2000 m. If these formations are oil-bearing, the carbon dioxide enables petroleum recovery. Byproduct heat from the plant can be used for district heating. Particularly when it is integrated with byproduct recovery, chemical synthesis of NH3 and H2SO4, and district heating, an integrated plant of this kind is an excellent example of a system of industrial ecology.
Fuel from Carbon Dioxide
An interesting potential use of carbon dioxide is to use it as a source of carbon for the synthesis of hydrocarbon fuels and other organic compounds including alcohols. The requirement for so doing is an abundant and inexpensive source of elemental hydrogen, H2, which can be reacted with CO2 through the reverse water-gas shift reaction:3
\[\ce{CO2 + H2 \rightarrow CO + H2O}\]
The CO produced can be reacted with additional H2 to produce methane gas (methanation), hydrocarbon liquids (Fischer-Tropsch synthesis), or alcohols. The overall process is the reverse of the energy-yielding combustion of hydrocarbon fuels, so it consumes a lot of energy and requires a cheap source of energy in order to be practical. The most likely energy source is “free” wind power generating electricity that can produce H2 gas by electrolysis of water (see Reaction 15.11.1). In this application the intermittent characteristic of wind power is not an issue and hydrogen produced in abundance during times of strong wind can be pumped underground to be withdrawn for subsequent reaction with CO2 and CO.


