15.8: Depletable Fossil Fuels
- Page ID
- 285396
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)Hydrocarbons from Wells
Currently, the most widely used sources of energy are liquid and gaseous hydrocarbons from underground. It is readily seen why liquid petroleum and natural gas became so popular because they are so easy to obtain from holes drilled into the ground, easy to transport by pipeline, and easy to use, especially in home furnaces (natural gas) and internal combustion engines (refined petroleum). But these sources suffer two major disadvantages: (1) They will eventually run out and (2) they contribute greenhouse gas carbon dioxide to the atmosphere.
Liquid petroleum occurs in pores of rock having a porosity of 10-30%, over half of which is normally occupied by water. Primary recovery typically removes about 30% of the crude oil. more advanced recovery techniques by injecting water or pressurized carbon dioxide can remove around 50% of the crude oil. A very sustainable technique is to burn petroleum in a pure oxygen atmosphere to generate electricity, collect the relatively pure CO2 product, and inject it back into the petroleum-bearing formation to remove more oil. By using advanced techniques that recover up to 60% of the available petroleum, oil fields that have been depleted by formerly used processes can be given a second life and yield as much more oil as they did originally.
The most exciting relatively recent development in utilizing hydrocarbons pumped from below ground consists of the development of methods to remove natural gas (CH4) from tight shale formations such as the natural-gas-rich Marcellus Shale deposits extending for 600 miles through sections of Virginia, West Virginia, Ohio, Pennsylvania, and New York. Methods have been developed for hydraulic fracturing (“hydrofracking”) these formations and enabling natural gas to flow by injecting water containing additives into the formations under very high pressures. Widespread development of these sources since approximately 2000 has led to a relative abundance of natural gas. Furthermore, since about 1990 natural gas has been extracted from coal seams, many of which are not suitable for mining. There are environmental issues involving water with both of these methods of natural gas extraction. There is some potential with hydrofracking shale formations to contaminate well water with natural gas and a search of the internet can bring up rather spectacular pictures of “burning water” in which natural gas coming out of a water faucet can be lit resulting in a substantial flame. Natural gas withdrawn from coal seams is usually mixed with copious quantities of water which in some cases is polluted.
Fossil Fuels Dug from Below Ground
Large quantities of fossil fuels are dug from below ground, either from underground mines or from surface pits. The largest source of petroleum that is imported into the U.S. is from tar sands in the Canadian province of Alberta. These are deposits of sand covered with heavy crude petroleum that are extracted from pits and carried by enormous trucks to locations where the oil is extracted by hot water or steam leaving immense quantities of relatively clean sand.
Another petroleum substitute that can be obtained by mining is shale oil, a material bound to oil shale rock in the form of complex organic material of biological origin called kerogen. Shale oil is removed from these rocks by heating in the absence of air. It is believed that as much as1.8.trillion barrels of shale oil could be recovered from deposits in Colorado, Wyoming, and Utah. The story is told that oil shale was discovered in this region by an earlier settler who constructed a fireplace and chimney in a cabin from the shale and was very much distressed when the first fire in the heating facility resulted in its burning, along with the surrounding cabin. Although large amounts of petroleum substitute could be recovered from oil shale, this resource is not likely to be developed on a large scale because the pyrolysis releases enormous amounts of greenhouse gas carbon dioxide and leaves a residue of ash from which salts such as sodium sulfate are readily leached. Furthermore, the liquid shale oil product contains a high content of potentially carcinogenic organonitrogen compounds.
Coal and Lignite
Coal and related solids are solid carbonaceous fossil fuels formed by the partial biodegradation of ancient biomass followed by geochemical processes involving heat and high pressures. Coal is differentiated largely by coal rank based upon percentage of fixed carbon, percentage of volatile matter, and heating value. Although “coal” gives the appearance of being pure carbon, it is actually a complex hydrocarbon-like material, typically with an empirical formula of around CH0.8 and containing from 1 to several percent sulfur, nitrogen, and oxygen. Of these elements, sulfur bound to the organic coal molecule and mixed with coal as mineral pyrite, FeS2, presents major environmental problems because of production of air pollutant sulfur dioxide during combustion. Much of the FeS2 can be removed physically from coal prior to combustion and sulfur dioxide can be removed from stack gas by various scrubbing processes. Lower rank brown coal and lignite typically have high moisture and bound oxygen contents. Most commonly, coal classified as brown coal is relatively closer in its constitution to the vegetation from which it was formed.
The greatest fraction of electricity production worldwide is from coal burned in boilers to raise steam that runs turbines connected to electrical generators. The potential is high for air pollution from this technique including especially fly ash and sulfur dioxide. These two pollutants are now generally well controlled. However, burning coal releases more greenhouse gas carbon dioxide per unit energy output than any other energy-yielding process and this release can only be controlled by extraordinary (and expensive) means.
Coal Conversion
A sustainable approach to utilizing the coal energy resource is coal conversion in which coal is converted to gaseous or liquid fuels or low-sulfur solids. Starting with the house of William Murdocks at Redruth, Cornwall, England, illuminated with coal gas in 1792, coal conversion has along history. Pall Mall in London was lit with gas from the first municipal coal-gas system in1807. The coal-gas industry began in the U.S. in 1816. The first coal gas plants operated by heating coal in the absence of air leaving a solid residue consisting mostly of carbon (that could be used as stove fuel) and produced a hydrocarbon-rich fuel especially effective for lighting. During the 1800s a gasification process was developed in which steam reacted with hot carbon to produce a mixture consisting primarily of H2 and CO (synthesis gas) to which it was necessary to add volatile hydrocarbons to make the fuel suitable for lighting. There were 11,000 coal gasifiers operating in the U.S. in the 1920s and the industry peaked in 1947 after which it was rapidly replaced by abundant natural gas sources. During World War II Germany made synthetic petroleum from synthesis gas, reaching a capacity of 100,000 barrels per day in 1944. The largest coal-based synthetic fuels plant operating today is in Sasol, South Africa, and now produces hydrocarbons and feedstocks equivalent to about 150,000 barrels of petroleum per day.
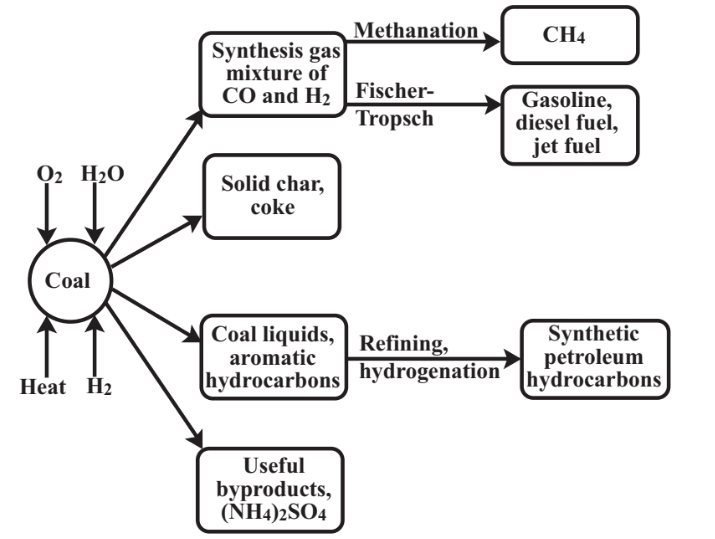
Although coal conversion could be developed as a substitute for petroleum, it is by no means a green process considering the environmental costs of coal mining, the production of toxic coal tar byproducts, and the enormous amounts of carbon dioxide generated during the conversion process. As discussed in the following section, the byproduct carbon dioxide can be captured and pumped underground where it can aid in petroleum recover.


