15.11: Renewable Energy Sources - Solar Energy
- Page ID
- 285730
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)Ideal energy sources are those that do not pollute and never run out. Such sources are commonly called renewable energy resources. There are several practical renewable energy resources that are discussed briefly in this section. The sun is the ultimate renewable energy source and solar energy is discussed first.
Solar Energy is The Best — When The Sun Shines
Sunshine comes close to meeting the criteria of an ideal energy source, including widespread availability, an unlimited supply, and zero cost up to the point of collection. The utilization of solar energy does not cause air, heat, or water pollution. Sunshine is intense and widely available in many parts of the world. If it were possible to collect solar energy with a collection efficiency of 10%, approximately one-tenth of the area of Arizona would suffice to meet U.S. energy needs, and at 30% collection efficiency, only about one-thirtieth of the area of that generally sunny state would suffice. But, keep in mind that such an area is still enormous and the implications of covering it with solar collectors would be profound.
There are several ways in which solar energy can be utilized. The simplest of these is for heating, and solar-heated houses and solar water heaters have been developed and used successfully. At a somewhat more sophisticated level, solar boilers have been developed that are located on towers and receive concentrated sunlight from an array of parabolic mirrors, thus generating steam to make electricity (see Figure 15.5). Some years ago a serious proposal was even made to use solar collectors in Earth orbit and convert the energy to a beam of microwave radiation focused on a receiver on Earth’s surface. Visions of this beam straying from its aiming point or hapless birds or even aircraft straying into it and being instantly cooked by an extraterrestrial microwave oven have prevented this plan from coming to fruition. Photosynthetic generation of biomass is another way of utilizing solar energy as discussed in a later section of this chapter.
Other than low-grade building and water heating, the most promising way to utilize solar energy is by its direct conversion to electricity in photovoltaic cells (see Figure 15.14). Originally just a laboratory curiosity, these devices became practical sources of electricity for satellites and space vehicles where their high cost was of little concern. But over the years they have become more efficient and cheaper, and it is now common to see arrays of these cells used to power data processors and signaling devices in remote locations. And some houses even have banks of photovoltaic cells.
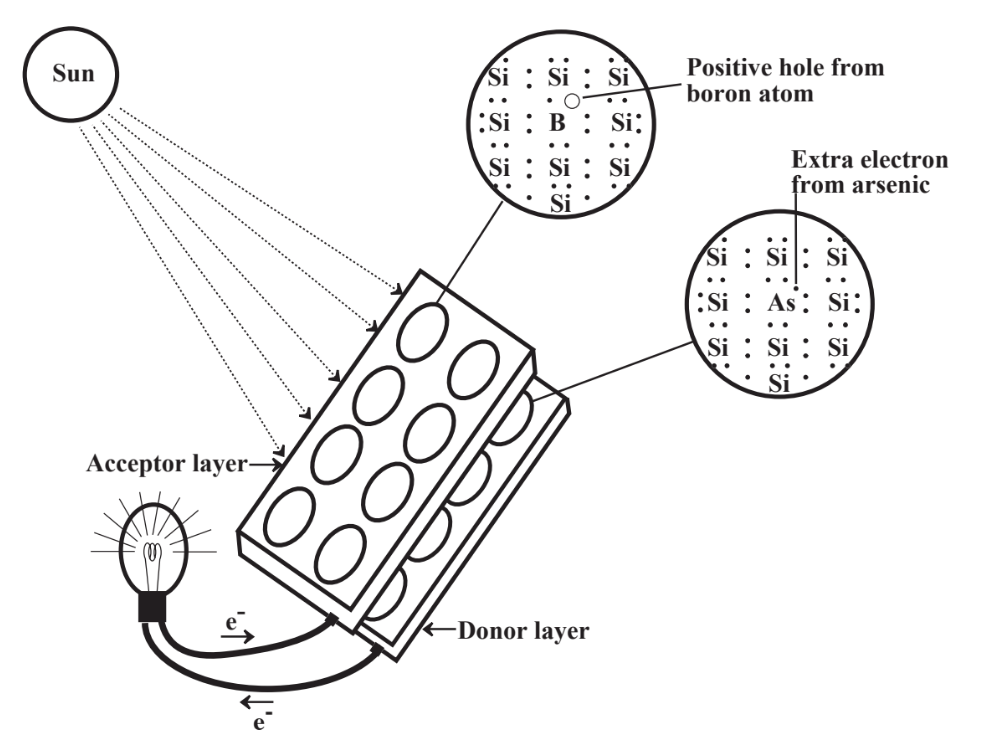
Photovoltaic cells depend upon the special electronic properties of silicon atoms containing low levels of other elements. The cell consists of two layers of silicon, a donor layer that is doped with about 1 part per million of arsenic atoms and an acceptor layer doped with about 1 part per million of boron. Examination of the Lewis symbols of these three elements,
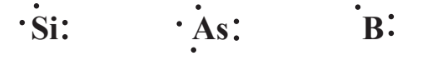
shows that substitution of an arsenic atom with its 5 valence electrons for a silicon atom with its 4 valence electrons in the donor layer gives a site with an excess of 1 electron whereas substitution of a boron atom with only 3 electrons for a silicon atom in the acceptor layer gives a site “hole” that is deficient in one electron. The surface of a donor layer in contact with an acceptor layer contains electrons that are attracted to the acceptor layer. When light shines on this area, the energy of the photons of light can push these electrons back onto the donor layer, from which they can go through an external circuit back to the acceptor layer. This flow of electrons constitutes an electrical current that can be used for energy.
Current photovoltaic cells are around 12–15% efficient in converting radiant solar energy to electricity at a cost significantly higher than that of electricity generated in fossil fuel powerplants. However, advances are continually being made in solar cell technology and it can be anticipated that efficiencies will continue to increase as costs decrease. The obvious major disadvantage of solar energy is that it does not work in darkness, and variable atmospheric conditions affect its output. Flexibility in electrical power grids allows such intermittent sources for up to 15% of power without using special devices for energy storage. Furthermore, there are means of storing energy, such as by extremely high-temperature/high-pressure supercritical water stored deep underground or mechanical energy stored in the extremely rapid rotation of flywheels.
A very attractive energy storage option for solar energy given the growing use of fuel cells is hydrogen gas. Electrolysis of water containing a solution of electrolyte (commonly KOH)
\[\ce{2H2O + electrical energy \rightarrow 2H2 (g) + O2 (g)}\]
with solar-generated electricity provides elemental hydrogen and oxygen, which are exactly the fuels used by fuel cells. Commercially available electrolyzers are 55-75% efficient in converting electricity to hydrogen and oxygen. The overall efficiency of this process can be increased significantly by the development of direct means for splitting water molecules into hydrogen and oxygen using the energy of light photons.


