15.13: Biomass Energy
- Page ID
- 285732
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)Biomass and liquid and gaseous fuels made by processing it are the most promising sources of renewable energy for transportation.4As noted earlier in this chapter, photosynthesis,
\[\ce{6CO2 + 6H2O (solar \: energy, } h \nu \ce{) \rightarrow C6H12O6 (glucose \: carbohydrate) + 6O2}\]
enables the conversion of solar energy to chemical energy in the form of biomass. Photosynthetically generated biomass supplies the food energy for essentially all organisms and,until about 200 years ago, was the source of most fuel. In addition to fuelwood and charcoal, biofuels, include forestry residues; agricultural byproducts; rapidly-growing grasses such as switchgrass; livestock manure; methane gas produced by anoxic fermentation of biomass; bioethanol from fermentation of sugar made from sugarcane, sugar beets, and cornstarch; and biodiesel synthesized from plant oils. As shown in Figure 15.6, photosynthesis suffers from the disadvantage of having less than 0.5% efficiency in the conversion of solar energy to chemical energy (although some plants, most notably sugarcane, convert solar energy to biomass energy with an efficiency of around 0.6%). Despite the limitations of photosynthesis in capturing energy, biomass is the predominant energy source in many developing regions of the world where approximately two billion people rely on wood for their primary household energy sources, especially for cooking. Today, more than 14% of the world’s primary energy comes from biofuels, especially fuelwood and charcoal from wood. Finland gets a significant portion of its energy from burning the black liquor byproduct from pulp and paper manufacture, which uses wood as a raw material.
Properly utilized, biomass is a largely nonpolluting source of energy. Since it is produced by photosynthesis, there is no net addition to global atmospheric carbon dioxide. Although the heating value of dried biomass is only about half that of coal, biomass combustion produces very little sulfur dioxide, and the ash residue containing mineral nutrients can be returned to soil without adding harmful heavy metals, which can be a problem with coal ash.
The kinds of biomass that can be used for fuel fall into the four categories of (1) lignocellulosic materials from perennial plants, crop residues, wood, and biowastes; (2) starch from corn and other grains; (3) sugars from sugarcane and sugarbeets; and (4) oils from soybeans, rapeseed, and palm oil. These potential sources are addressed here.
As shown in Reaction 15.13.1, carbohydrates, such as glucose, C6H12O6, are produced by photosynthesis. They can be burned directly, converted chemically to other fuels, or fermented to produce ethyl alcohol fuel. Hydrocarbons are more desirable as fuels, and some plants produce them directly. One example is the Philippine plant, Pittsosporum reiniferum, the fruits of which contain such a high content of hydrocarbon terpenes, primarily α-pinene and myrcene, that they can be burned to provide illumination. Rubber trees and other plants, such as Euphorbia lathyrus (gopher plant), a small bush growing wild in California, produce hydrocarbon emulsions. Seed oils, such as those produced by sunflowers and peanuts, and more exotic sources including buffalo gourd, cucurbits, and Chinese tallow tree, can be used for fuel, especially in diesel engines.
Despite concerns that not enough biomass can be grown to produce fuel and that it detracts from food supplies, it should be noted that about 150 billion metric tons of biomass are produced in the world each year by photosynthesis, mostly from uncontrolled plant growth. Corn, the most productive common field crop produces about 4 metric tons per acre of dry biomass annually(including stalks, leaves, husks, and corncobs). Switchgrass, a prolific producer of biomass, typically generates 11-12 tons of biomass per acre per year (there are 640 acres in a square mile of land). About 6% of the biomass generated globally each year would be equivalent to the world’s demand for fossil fuels. Cultivation for fuel biomass of 6-8% of the land area of the 48 contiguous states would provide energy equivalent to annual U.S. consumption of petroleum and natural gas. Furthermore, only a small fraction of widely grown grain crops goes into grain; the rest is plant biomass, much of which could be used for energy production. And the U.S. has vast areas of underutilized land that could be devoted to the cultivation of energy-yielding plants. Much of this neglected, erosion-prone land would benefit from the cultivation of perennial plants that could be harvested for energy and regrow from roots left in the ground, thus lowering water and wind erosion.
A potentially important aspect of biomass fuel production that could improve its economics and environmental acceptability is coproduction of protein that can be used to feed animals. Now most protein used for feed comes from grain, particularly soybeans and corn. However, legumes(especially alfalfa) and perennial and annual grasses generate significant amounts of leaf protein. This material can be isolated by squeezing protein-rich juice from freshly ground plant leaves and heating the juice to precipitate the protein. The remaining fibrous material can then be used as a feedstock for the synthesis of biofuels.
Prolific Production of Biomass from Algae
Microscopic single-cell algae (microalgae) and photosynthetically-capable bacteria(cyanobacteria) growing in water can readily produce 10 times more biomass per unit area than terrestrial plants. In addition to their prolific productivity, microalgae offer several potential advantages for the generation of biofuels. These advantages include high production of oils and lipids (30-60% of dry algae mass), ability to grow in areas not suitable for terrestrial plants, ability to grow in saline water including seawater, growth in nutrient-rich sewage, and ability to grow in water enriched in dissolved carbon dioxide, such as from combustion sources. Concentrated by centrifugation and suspended in water or other liquid, microalgae can be introduced as a fluid emulsion into biorefineries offering processing advantages over soli.d biomass sources. Because of these advantages, microalgae are likely to become the predominant source of biofuels in the future.
Fuels from Fermentation of Biomass
Biological fermentation can be used to produce fuels from biomass. Yeasts act upon carbohydrates,
\[\ce{C6H12O6 \rightarrow 2C2H5OH + 2CO2}\]
to produce ethanol, C2H5OH. This liquid alcohol can be used alone as a fuel, but is usually added to gasoline at levels of about 10% to produce gasohol, which burns more cleanly and with less CO output than ordinary gasoline. The source of carbohydrate for ethanol production is usually corn grain or sugar produced by sugarcane. Grain-based ethanol has seen strong growth in the U.S. and some other countries in recent years, much of it due to legislative initiatives. However, the net energy yield from this source is very low and its production competes with food crops, so it is nota very sustainable means of producing fuel. Ethanol from sugarcane sugars is significantly more competitive in Brazil, a prolific producer of sugarcane.
Another biomass fermentation occurs with methane-forming bacteria,
\[\ce{C6H12O6 \rightarrow 3CH4 + 3CO2}\]
to produce methane gas, CH4. The gas mixture produced by this biochemical process can be burned directly, or the carbon dioxide can be removed to produce pure methane gas. Anoxic(oxygen-free) methane digesters used to degrade the biomass in sewage sludge, the residue from biological treatment of wastewater, can generate enough power to provide for the pumping and electrical needs of a large sewage treatment plant. Small methane digesters running on crop and food residues and human and animal wastes are used in rural areas of China to provide methane for cooking and lighting.
Biodiesel Fuel
Biodiesel fuel is a combustible liquid synthesized from lipids, primarily those from plant oil seeds such as soybeans. Diesel fuel from petroleum consists of high-molecular-mass hydrocarbons containing 10-20 C atoms generally in a straight chain. Most lipids from plants are fatty acid esters of glycerol, such as triglyceride of stearic acid shown in Chapter 7, Figure 7.5. Examination of this formula shows that the fatty acid entities in it are predominantly straight-chain hydrocarbons except for the oxygen-containing carboxylate groups through which they are attached to the glycerol alcohol. Hydrolysis of the triglyceride and esterification with methanol yields liquid esters such as methyl stearate,
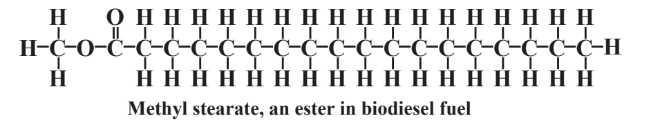
that have the combustion characteristics needed for diesel fuel. In addition to methyl stearate, biodiesel fuel contains methyl esters of other fatty acids including linoleic acid, oleic acid, lauric acid, and behenic acid. Unlike ethanol, which cannot be transported through existing pipeline systems because it absorbs water, is therefore corrosive, and must be blended with gasoline at the point of distribution, biodiesel can be handled through existing facilities.
Biodiesel fuel can be synthesized from oils extracted from rapeseed (the major source in Europe), soybean (predominant in the U.S.), sunflower, palm, coconut, and jatropha. Both rapeseed and soybeans leave a protein-rich byproduct after the oil has been removed that is a good food source for animals. Palm oil, coconut oil and jatropha (from Jatropha curcus, planted for hedges) are attractive oil sources for biodiesel production because they come from perennials that thrive in the tropics. Unfortunately, the explosive growth of palm oil tree plantations in Malaysia and Indonesia has resulted in high levels of rain forest destruction.
As noted above, microalgae are prolific producers of biomass and these organisms may have oil contents exceeding 50% making algae attractive for the production of biodiesel fuel. Whereas the annual production of biodiesel from soybeans may reach 200 liters per acre and from palm oil 2,500 liters per acre, optimistic projections for biodiesel production from algae are as much as 40,000 liters per acre annually. Furthermore, as noted above, algae can be produced on desert lands and in saltwater, thus not competing with food crops.


