15.6: Green Technology for Energy Conversion
- Page ID
- 285394
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)Green technologies have much to do with the important processes by which energy is converted between various forms. Some of the more important aspects of such conversions are discussed here.
Energy Conversion Efficiency
Energy is best conserved by efficient energy conversion. Vastly improved energy conversion efficiencies have been achieved in heat engines such as automobile engines and gas turbines by higher combustion temperatures made possible by improved materials and heat-resistant lubricating oils. Computerized design and operation of engines enabling optimum ignition timing, valve timing, and fuel injection have made possible extremely efficient engines.
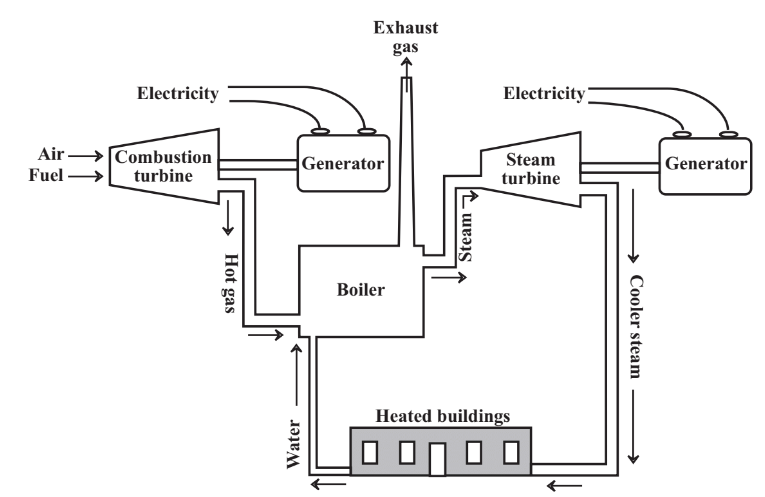
As noted in the discussion of the Carnot equation above, heat engines typically dissipate more than half of the energy in fuel as heat. A small fraction of this heat is used by heaters in automobiles. In stationary power plants much of this energy can be reclaimed for heating buildings or chemical processes with combined power cycles. as illustrated in Figure 15.7. Typically, in combined power cycle installations gas or fuel oil is burned in a turbine engine that is much like the engine of a turboprop airplane, and the rotating shaft of this engine is coupled to a generator to produce electricity. The hot exhaust gases from the combustion turbine can be injected into a boiler where their heat turns liquid water to steam. This steam can be run through a steam turbine coupled to a generator to produce more electricity. Steam leaving the steam turbine still contains a lot of heat, and can be conveyed to homes and other structures for heating. The water condensed from this steam is pure and is recycled to the boiler, thus minimizing the amount of makeup boiler feedwater, which requires expensive treatment to make it suitable for use in boilers. Such a system as the one described is in keeping with the best practice of industrial ecology. Heating with steam that has been through a steam turbine, a concept known as district heating, is commonly practiced in Europe (and many university campuses in the U.S.) and can save large amounts of fuel otherwise required for heating.
Conversion Efficiency of Chemical Energy
In some cases a need exists to convert chemical energy from one form to another so that it can be used in a desired fashion. The generation of hydrogen gas from fossil fuels is an important chemical energy conversion process that may become much more widely practiced as fuel cells, which use elemental hydrogen as a fuel, come into more common use. Hydrogen can be obtained from a number of sources. The cheapest and most abundant raw material for hydrogen generation is coal and the same general processes can be applied to other carbon-containing materials, especially renewable biomass. When carbon-based materials are used to generate hydrogen, the hydrogen actually comes from steam. In this process, known as coal gasification part of the coal is burned in an oxygen stream,
\[\ce{C(coal) + O2 \rightarrow CO2 + heat}\]
leaving a solid residue of very hot carbon from the unburned coal. This material reacts with water in steam,
\[\ce{C(hot) + H2O \rightarrow H2 + CO}\]
to generate elemental H2 and CO in a reaction that absorbs heat. The CO can be reacted with more steam over an appropriate catalyst,
\[\ce{CO + H2O \rightarrow H2 + CO2}\]
to increase the ratio of H2 to CO.
The reactions shown above for the generation of elemental hydrogen from coal and water have been used for well more than a century in the coal gasification industry. Before natural gas came into common use, steam blown over heated carbon was used to generate a synthesis gas mixture of H2 and CO that was piped into homes and burned for lighting and cooking. The mixture burned well, but, in addition to forming treacherous explosive mixtures with air, it was lethal to inhale because of the toxic carbon monoxide. But the process may have a future for the generation of elemental hydrogen for use in fuel cells. By using pure oxygen as an oxidant, it raises the possibility of producing greenhouse gas carbon dioxide in a concentrated form that can be pumped underground or otherwise prevented from getting into the atmosphere. Retention of carbon dioxide in this manner is called carbon sequestration and is the subject of some intense research.
The synthesis gas mixture of H2 and CO2 is a good raw material for making other chemicals, including hydrocarbons that can be used as gasoline or diesel fuel. Combined in the correct ratios over a suitable catalyst, these two gases can be used to make methane, the main constituent of natural gas:
\[\ce{CO + 3H2 \rightarrow CH4 + H2O}\]


