15.5: Conversions Between Forms of Energy
- Page ID
- 285393
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)The most abundant sources of energy are usually not directly useful and must be converted to other forms. Therefore, much of what is done with energy involves changing it from one form to another. As an example, the nuclear energy that can be extracted from a few kilograms of natural uranium is enormous. But in order to get any benefit from it, the uranium must first be enriched in the isotope whose nucleus can undergo fission (split) to release the energy, the enriched uranium must be placed in a nuclear reactor where fission occurs, converting the nuclear energy to heat, this heat is used to produce steam, the steam is run through a turbine to produce mechanical energy, and the turbine is coupled to a generator to convert its mechanical energy to electrical energy. The various conversions of energy from one form to another occur with different efficiencies. The successful practice of industrial ecology tries to maximize the efficiencies of energy conversion.
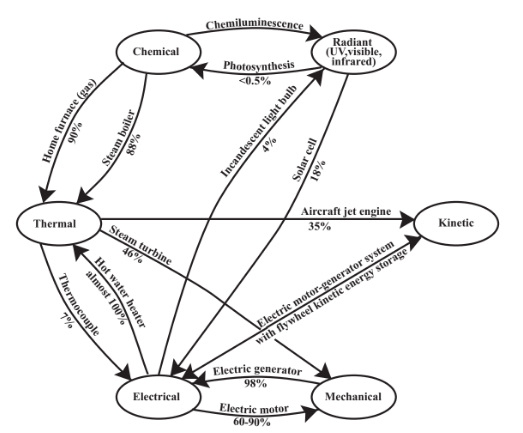
Figure 15.5 shows some of the most common devices for converting energy from one form to another and Figure 15.6 illustrates important energy conversions along with the percentage efficiencies with which some of these conversions can be carried out. Examination of the different percentage efficiencies for energy conversion given in Figure 15.6 shows differences ranging from very low to almost 100%. But they point to areas in which improvements may be sought. For example, photosynthesis is less than about 0.5% efficient in converting light energy to chemical energy. Despite this dismal figure, photosynthesis has generated the fossil fuels from which industrialized societies now get their energy and provides a significant fraction of energy in areas where wood and agricultural wastes are used. The intriguing possibility is suggested that genetically modified plants may be developed with much higher photochemical efficiencies, leading to greatly increased use of renewable biomass as an energy source. The poor efficiency of conversion of electricity to light in the incandescent light bulb points to the need to replace these wasteful devices with fluorescent bulbs that are 5 or 6 times more efficient.
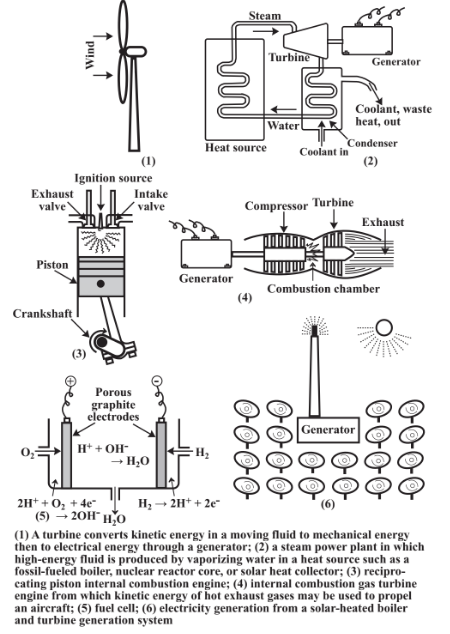
The most common kind of energy conversion carried out in the anthrosphere is the conversion of heat, produced by chemical combustion processes, to mechanical energy used to propel a vehicle or run an electrical generator. This occurs, for example, when gasoline in a gasoline engine burns, generating hot gases that move pistons in the engine connected to a crankshaft that converts the up-and-down movement of the pistons to rotary motion that drives a vehicle’s wheels. It also occurs when hot steam generated at high pressure in a boiler flows through a turbine connected directly to an electrical generator. Unfortunately, the laws of thermodynamics dictate that the conversion of heat to mechanical energy is always much less than 100% efficient. The Carnot equation,
\[\textrm{Percent efficiency} = \frac{T_{1} -T_{2}}{T_{1}} \times 100}\]
states that the percent efficiency is a function of the inlet temperature (for example, of steam), T1, and the outlet temperature, T2, both expressed in Kelvin ( ̊C + 273). Consider a steam turbine in which steam impinges on vanes attached to a rapidly rotating shaft. If the inlet temperature is 850K and the outlet temperature is 330 K, substitution into the Carnot equation gives a maximum theoretical efficiency of 61%. An inability to introduce all the steam at the highest temperature combined with friction losses of energy reduce the energy conversion efficiency of most modern steam turbines to just below 50%. Since only about 80% of the chemical energy used to raise steam by combustion of fossil fuel in a boiler is actually transferred to water to produce steam, the net efficiency for conversion of chemical energy in fossil fuels to mechanical energy to produce electricity is about 40%. Fortunately, essentially all the mechanical energy in a rotating turbine can be converted to electricity in the generator to which it is connected, so the overall efficiency of conversion of fossil fuel chemical energy to electricity is about 40%. The conversion of nuclear energy to mechanical energy in a reactor-powered steam turbine is only about 30% because reactor peak temperatures are limited for safety reason.
Another example of the application of the Carnot equation is provided by the internal combustion piston engine shown in Figure 15.5(3) in which a complete cycle consists of (1) a downward stroke sucking air into the cylinder, (2) a compression stroke during which fuel is injected, (3) ignition of the air/fuel mixture forcing the piston down, and (4) an exhaust stroke in which the exhaust gases are forced out through the open exhaust valve as the piston moves upward. The efficiency of the internal combustion engine increases with the peak temperature reached by the burning fuel, which increases with the degree of compression during the compression stroke. This temperature is highest for the diesel engine in which the compression is so high (up to around 20:1) that fuel injected into the combustion chamber ignites without a sparkplug ignition source. Whereas a standard gasoline engine is typically about 25% efficient in converting chemical energy in fuel to mechanical energy, a diesel engine is typically 37%efficient, with some reaching higher values.
Fuel Cells
Fuel cells convert the energy released by electrochemical reactions directly to electricity without going through a combustion process and electricity generator. Fuel cells are the primary means for utilizing hydrogen fuel and are becoming more common as electrical generators. The electrode reactions in a fuel cell are shown in Figure 15.5(5) and the net reaction is
\[\ce{2H2 + O2 \rightarrow 2H2O + electrical energy}\]
the only product of which is water. A number of different fuel cell types are at various stages of development.1 Development is underway of solid-oxide fuel cells, operating around 1000 ̊C that produce an exhaust that is hot enough to drive a turbine or cogenerate steam. with steam cogeneration, such systems may be able to develop overall efficiencies of up to 80%


