13.11: Catalysts and Catalysis
- Page ID
- 285716
The main components of a chemical process can be divided into the categories of catalysts, media, feedstocks, and reagents, all of which are important in the practice of green chemistry. Catalysts and catalysis are addressed in this section and the other aspects of chemical production are addressed in Sections 13.13 and 13.14.
An ideal green chemical reaction occurs with 100% atom efficiency using only reactants and no other reagents under mild conditions with only moderate input of thermal energy and without any catalysts. Unfortunately, few chemical processes meet these criteria.
In the past, especially in the synthesis of fine chemicals and pharmaceuticals where the objective has been to simply make the desired product without much consideration of waste, so-called stoichiometric reagents have been used. These have included inorganic oxidants such as MnO2, KMnO4, K2Cr2O7; metal reductants including zinc, magnesium, sodium, and iron; and metal hydride reducing agents, especially LiAlH4 and NaBH4. Various organic reactions including sulfonations and nitrations employ Lewis acids (BF3, AlCl3, ZnCl2) and mineral acids (HF, H2SO4, and H3PO4). Many of these processes are indirect methods of adding to organic molecules hydrogen (reduction), oxygen (oxidation), carbon, and nitrogen. A problem with these reagents is the large amount of inorganic wastes produced. Where possible, it is much more desirable to employ catalysts to attach the simplest possible forms of the elements including H2 for reduction, O2 or H2O2 for oxidation, CO or CO2 for attachment of carbon, and NH3 for attachment of N.
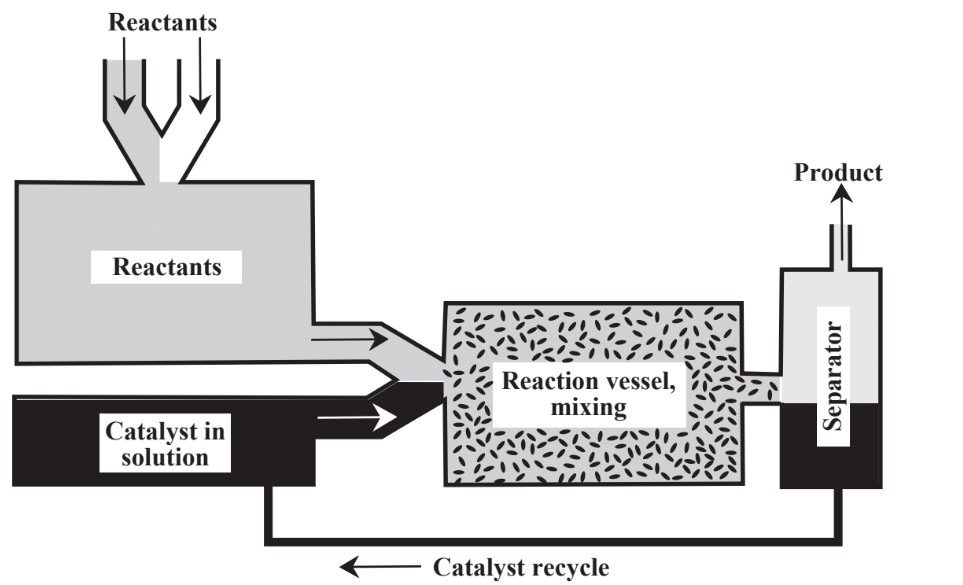
Instead of relying only on stoichiometric reagents, catalysts are commonly employed. As discussed in Chapter 5, Section 5.5, catalysts, are substances that enable reactions to proceed at significant rates without themselves being consumed. Catalysts are of great importance in the practice of green chemistry for a number of reasons including their ability to facilitate reactions and to reduce the energy required to enable reactions to proceed. There are two major approaches to contacting catalyst with a reaction mixture as shown in Figure 13.4. In heterogeneous catalysis the catalyst is held on a support and the reactants flow over it. In homogeneous catalysis the
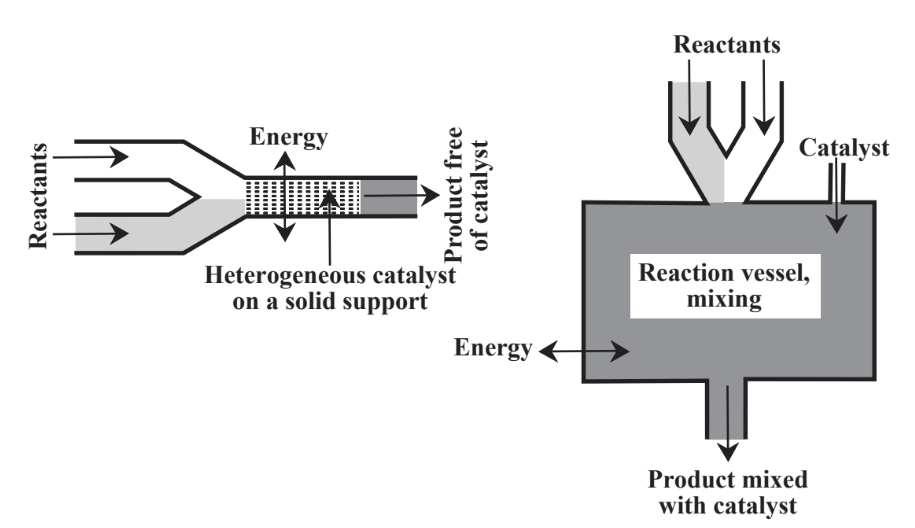
catalyst is placed in the reaction mixture and either remains in the product or is separated from the product in a separate step. In general, homogeneous catalysts have high activity and selectivity whereas heterogeneous catalysts are more easily recovered and recycled. Much of the activity in green chemistry has been in the area of catalysis, especially in the development of heterogeneous catalysts that do not contaminate the product and that can be reused multiple times. Large numbers of different catalysts are used in chemical processes and their potential toxicities, production of byproducts and contaminants, recycling, and disposal are matters of considerable importance in the chemical industry.
An important area of endeavor in the development of improved catalysts with respect to green chemistry is selectivity enhancement. Basically, this means developing a catalyst that is very selective in what it does, ideally making the right product and nothing else. A highly selective catalyst increases the percentage utilization of raw material (increased percent yield) and decreases the amount of waste byproducts from undesired side reactions.
Another important attribute of a good catalyst is related to the basic way in which a catalyst works, which is by lowering the activation energy that is required to make a reaction proceed at a significant rate. As a consequence, catalysts lower the total amount of energy that must be put into a chemical process to get it to occur. Lowered energy requirements are a basic part of the practice of green chemistry and in this respect highly efficient catalysts can be extremely beneficial in reducing energy consumption and in so doing lowering costs and environmental impact.
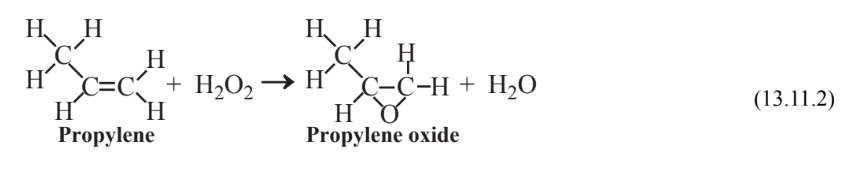
Numerous green industrial chemical reactions have been developed in recent years, most employing some sort of catalyst. An example is the synthesis of the widely used industrial chemical propylene oxide starting with elemental H2 and O2. Around 1985 it was discovered that propylene could be oxidized to propylene oxide with 30% hydrogen peroxide using a titanium silicate catalyst, but the process was not economic because of the high cost of hydrogen peroxide. A Green Chemistry Presidential Challenge prize was awarded in 2007 for an economical process to make hydrogen peroxide by combining H2 and O2 in a gas mixture at levels of H2 directly below the lower flammability limit of H2 using a catalyst composed of palladium-platinum nanoparticles:
\[\ce{H2 + O2 \rightarrow 2H2O2}\]
As a second step in the process the hydrogen peroxide is reacted with propylene to produce propylene oxide with water as the only byproduct: