12.4: Metabolism and Control in Organisms
- Page ID
- 285367
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)As discussed in Section 7.8 in respect to the processing of biochemicals, living organisms continually process materials and energy, a process called metabolism. Photosynthesis, which is mentioned above, is the metabolic process that provides the base of the food chain for most organisms. Animals break down complex food materials to smaller molecules through the process of digestion. Respiration occurs as nutrients are metabolized to yield energy:
\[\ce{C6H12O6 (glucose) + 6O2 \rightarrow 6CO2 + 6H2O + energy}\]
Organisms assemble small molecules to produce biomolecules, such as proteins, by a synthesis process.
In addition to viewing metabolism as a phenomenon within an individual organism, it can be viewed as occurring within groups of organisms living in an ecosystem. Consider, for example, the metabolism of nitrogen within an ecosystem. Elemental nitrogen from the atmosphere may be fixed as organic nitrogen by bacteria living symbiotically on the roots of leguminous plants, then converted to nitrate when the nitrogen-containing biomass decays. The nitrate may be taken up by other plants and incorporated into protein. The protein may be ingested by animals and the nitrogen excreted as urea in their urine to undergo biological decay and return to the atmosphere as elemental nitrogen. Carbon from carbon dioxide in the atmosphere may be incorporated into biomass by plant photosynthesis, then eventually returned to the atmosphere as carbon dioxide as the biomass is used as a food source by animals.
Enzymes in Metabolism
In Chapter 5, Section 5.5, catalysts were defined as materials that enable a reaction to occur without themselves being consumed. Living organisms have catalysts that are very important in metabolism. These catalysts are special proteins that enable biochemical reactions to take place called enzymes. Enzymes speed up metabolic reactions by as much as almost a billion-fold. In addition to making reactions go much more rapidly, enzymes are often highly specific in the reactions that they catalyze. The reason for the specificity of enzymes is that they have very specific structures that fit with the substances upon which they act.
Enzymes were discussed in Chapter 7, Section 7.7, and their action illustrated in Figure 7.9 with respect to their processing of biochemicals. The first step in the function of enzymes is the reversible formation of an enzyme/substrate complex that forms because of the complementary shapes of the enzyme (more specifically the active site on the enzyme) and the substrate. The second step is the formation of products accompanied by release of the unchanged enzyme molecule. A very common enzymatic process called hydrolysis involves splitting a molecule accompanied by the addition of water with an H atom going to one of the products and an OH group to the other. Other types of enzyme-catalyzed reactions occur, including the joining of two molecules, modifications of organic functional groups on substrate molecules, and rearranging the structures of molecules.
The names of enzymes, usually ending in “-ase” often reflect their functions and may also indicate where they operate. An example is gastric proteinase, a name that indicates the enzyme acts in the stomach (gastric) and hydrolyzes proteins (proteinase). The enzyme released by the pancreas that hydrolyzes fats is called pancreatic lipase.
A number of factors can affect enzyme action. One important factor is temperature. Organisms without temperature-regulating mechanisms have enzymes that increase in activity as temperature increases up to the point where the heat damages the enzyme, after which the activity declines precipitously with increasing temperature. Enzymes in mammals function optimally at body temperature (37 ̊C for humans) and are permanently destroyed by about 60 ̊C. There is particular interest in enzymes that function in bacteria that live in hot springs and other thermal areas where the water is at or near boiling. These enzymes may turn out to be very useful in commercial biosynthesis operations where the higher temperature enables reactions to occur faster. Acid concentration also affects enzymes, such as those that function well in the acidic environment of the stomach, but stop working when discharged into the slightly basic environment of the small intestine (were this not the case, they would tend to digest the intestine walls).
A significant concern with potentially toxic substances is their adverse effects upon enzymes. As an example, organophosphate compounds, such as insecticidal parathion and military poison sarin “nerve gas” bind with acetylcholinesterase required for nerve function, causing it not to act and stopping proper nerve action. Some substances cause the intricately wound protein structures of enzymes to come apart, a process called denaturation, which stops enzyme action. The active sites of enzymes at which substrates are recognized have a high population of -SH groups. Heavy metals, such as lead and cadmium, have a strong affinity for−SH groups and may bind at enzyme active sites thus destroying the function of the enzymes.
Enzymes are of significant concern in the practice of green chemistry. One obvious relationship is that between enzymes and chemicals that are toxic to them. In carrying out green chemical processes, such chemicals should be avoided wherever possible. Another obvious relationship has to do with the use of biological processes to perform chemical operations, which are usually done under much milder and environmentally friendly conditions biologically than chemically. Biochemical processes are all carried out by enzymes. For example, several enzymes, starting with hexokinase, are involved in the multistepped biochemical fermentation synthesis of ethyl alcohol from carbohydrate glucose. With recombinant DNA technology (see Section 12.8) it is now possible to invest bacteria with enzyme systems from other organisms designed to carry out desired biochemical processes. Bacteria are much more amenable to handling and usually much more efficient than the organisms from which the genes for the desired enzyme systems are taken. Another approach is to use isolated enzymes immobilized on a solid support to carry out biochemical processes without the direct involvement of an organism.
Nutrients
The raw materials that organisms require for their metabolism are nutrients. Those required in larger quantities include oxygen, hydrogen, carbon, nitrogen, phosphorus, sulfur, potassium, calcium, and magnesium and are called macronutrients. Plants and other autotrophic organisms use these nutrients in the form of simple inorganic species, such as H2O and CO2, which they obtain from soil, water, and the atmosphere. Heterotrophic organisms obtain much of the macronutrients that they need as carbohydrates, proteins and lipids (see Chapter 7) from organic food material.
An important consideration in plant nutrition is the provision of fertilizers consisting of sources of nutrient nitrogen, phosphorus, and potassium. A large segment of the chemical manufacturing industry is involved with fixing nitrogen from the atmosphere as ammonia, NH3, and converting it to nitrate (NO3-), urea (CON2H4), or other compounds that are applied to the soil as nitrogen fertilizer. Phosphorus is mined as mineral phosphate that is converted to biologically available phosphate (H2PO4- and HPO42- ions) by treatment with sulfuric or phosphoric acid. Potassium is mined as potassium salts and applied directly as fertilizer. The ongoing depletion of sources of phosphorus and potassium fertilizer is a sustainability issue of significant concern.
Organisms also require very low levels of a number of micronutrients, which are usually used by essential enzymes that enable metabolic reactions to occur. For plants, essential micronutrients include the elements boron, chlorine, copper, iron, manganese, sodium, vanadium, and zinc. The bacteria that fix atmospheric nitrogen required by plants require trace levels of molybdenum. Animals require in their diet elemental micronutrients including iron and selenium as well as micronutrient vitamins consisting of small organic molecules.
Control in Organisms
Organisms must be carefully regulated and controlled in order to function properly. A major function of these regulatory functions is the maintenance of the organism’s homeostasis, its crucial internal environment. The most obvious means of control in animals is through the nervous system in which messages are conducted very rapidly to various parts of the animal as nerve impulses. More advanced animals have a brain and spinal cord that function as a central nervous system (CNS). This sophisticated system receives, processes, and sends nerve impulses that regulate the behavior and function of the animal. Effects on the nervous system are always a concern with toxic substances. For example, exposure to organic solvents that dissolve some of the protective lipids around nerve fibers can lead to a condition in which limbs do not function properly called peripheral neuropathy. Therefore, a major objective of green chemistry is to limit the use of and human exposure to such solvents.
Both animals and plants employ molecular messengers that move from one part of the organism to another to carry messages by which regulation occurs. Messages sent by these means are much slower than those conveyed by nerve impulses. Molecular messengers are often hormones discussed as lipids in Section 7.5. Hormones are carried by a fluid medium in the organism, such as the bloodstream, to cells where they bind to receptor proteins causing some sort of desired response. For example, the process may cause the cell to synthesize a protein to counteract an imbalance in homeostasis. Some hormones called pheromones carry messages from one organism to another. They commonly serve as sex attractants. Some biological means of pest control use sex pheromones to cause sexual confusion in pesticidal insects, thus preventing their reproduction. Figure 12.2 shows a common plant hormone and a common animal hormone.
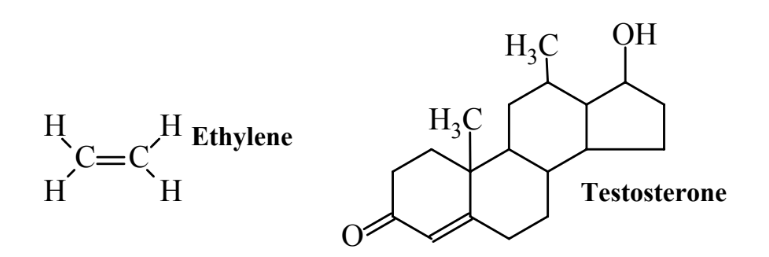
In animals, regulatory hormones are commonly released by endocrine glands shown for humans in Chapter 7, Figure 7.6. Endocrine glands in humans include the anterior pituitary gland that releases human growth hormone, the parathyroid gland that releases a hormone to stimulate uptake of calcium into the blood from bones and the digestive tract, and the pancreas that release insulin to stimulate glucose uptake from blood. These hormones are carried to target cells in fluids external to the cells. A significant concern with toxic substances is their potential to interfere with the function of endocrine glands. Another concern is that some toxic substances may mimic the action of hormones. For example, evidence exists to suggest that premature sexual development in some young female children can be caused by ingestion of synthetic chemicals that mimic the action of the female sex hormone estrogen.


