11.9: Plant Nutrients and Fertilizers in Soil
- Page ID
- 285699
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)Plant biomass is composed largely of carbon, hydrogen, and oxygen, which plants extract from water and atmospheric carbon dioxide. Other nutrients that plants require in relatively large quantities are calcium, magnesium, and sulfur, which are usually in sufficient abundance in soil, and nitrogen, phosphorus, and potassium, which are commonly added to soil as fertilizers.
Soil acidity in the form of H+ ion builds up as plant roots exchange H+ for other cationic nutrients in soil. When acidity reaches excessive levels, the soil is no longer productive. Acidity can be neutralized by the addition of lime (CaCO3), which neutralizes acidity according to the following reaction:
\[\ce{Soil (H^{+})_2 + CaCO3 \rightarrow Soil) Ca^{2+} + CO2 + H2O}\]
This process also adds calcium to soil.
Essential plant nutrient nitrogen is very much involved with nature’s nitrogen cycle, which is significantly modified by human activities. Major aspects of this cycle are the following:
- At 79% N2, Earth’s atmosphere constitutes an inexhaustible nitrogen resource, although, because of the extreme stability of the N2 molecule, it is difficult to extract nitrogen from air in a chemically combined form.
- Rhizobium bacteria growing on the roots of leguminous plants, such as clover and soybeans, convert atmospheric nitrogen to nitrogen chemically bound in biomolecules. This nitrogen is converted to ammonium ion, NH4+, when plant residues and animal feces, urine, and carcasses undergo microbial decay.
- Lightning and combustion processes convert atmospheric nitrogen to nitrogen oxides, and ammonia manufacturing plants produce NH3 from atmospheric elemental nitrogen and elemental hydrogen produced by natural gas.
- Soil microbial processes oxidize ammoniacal nitrogen (NH4+) to nitrate ion, NO3-, the form of nitrogen most readily used by plants. Microbial processes also produce gaseous N2 and NO2 which are released to the atmosphere, a process called denitrification that completes the nitrogen cycle.
Natural processes usually do not produce sufficient nitrogen to allow maximum plant growth, so that artificial means are used to extract nitrogen in a chemically combined form from the atmosphere. This is done by the Haber process combining elemental N2 and H2 over a catalyst at very high pressures of about 1000 times atmospheric pressure and an elevated temperature of 500 ̊C. The reaction is
\[\ce{N2 + 3H2 \rightarrow 2NH3}\]
producing ammonia that is 82% chemically bound N. This anhydrous ammonia can be applied directly below the soil surface where its tremendous attraction to soil moisture binds it to the soil. It can also be applied as a 30% solution of NH3 in water, and is sometimes added directly to irrigation water. Ammonia, which is held in soil as ammonium ion, NH4+, is not well assimilated directly by most plants. But it is slowly oxidized by the action of soil bacteria using atmospheric O2 oxidant to nitrate ion, NO3-, which is used directly by plants.
A solid form of nitrogen fertilizer can be made by reacting ammonia with oxygen over a platinum catalyst to make nitric acid, HNO3, and reacting the acid with basic ammonia to make ammonium nitrate, NH4NO3. This molten material is solidified into small pellets that can be applied to soil as fertilizer. Ammonium nitrate mixed with fuel oil is used for blasting to quarry rock, and it was the explosive used in the bombing of the Oklahoma City Federal Building in 1995. A safer alternative to ammonium nitrate as a solid nitrogen fertilizer is urea, which is made by a process that, overall, involves the reaction of carbon dioxide and ammonia:
\[\ce{CO2 + 2NH3 \rightarrow CO(NH2)2 + H2O}\]
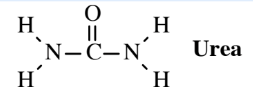
Phosphorus is an essential plant nutrient required for cellular DNA and other biomolecules. Itis utilized by plants as H2PO4- and HPO42- ions. Phosphate minerals that can be used to manufacture phosphorus-containing fertilizers occur in a number of places throughout the world. In the United States, Florida has especially abundant phosphate resources, largely as fluorapatite, Ca5(PO4)3F, as well as hydroxyapatite, Ca5(PO4)3OH. These phosphate minerals are too insoluble to serve directly as fertilizers and are treated with phosphoric acid and sulfuric acid to make superphosphates that are much more soluble and available to plants:
\[\ce{2Ca5(PO4)3F(s) + 14H3PO4 + 10H2O \rightarrow 2HF(g) + 10Ca(H2PO4)2 \cdot H2O}\]
\[\ce{2Ca5(PO4)3F(s) + 7H2SO4 + 3H2O \rightarrow 2HF(g) + 3Ca(H2PO4)2 \cdot H2O + 7CaSO4}\]
Potassium as the potassium ion, K+, is required by plants to regulate water balance, activate some enzymes, and enable some transformations of carbohydrates. Potassium is one of the most abundant elements in the earth’s crust, of which it makes up 2.6%; however, much of this potassium is not easily available to plants. For example, some silicate minerals such as leucite, K2O•Al2O3•4SiO2, contain strongly bound potassium. Exchangeable potassium held by clay minerals is relatively more available to plants. Potassium for fertilizer is simply mined from the ground as salts, particularly KCl, or pumped from beneath the ground as potassium-rich brines. Large potassium deposits occur in the Canadian province of Saskatchewan.
Plants require several micronutrients, largely elements that occur only at trace levels, for their growth. These include boron, chlorine, copper, iron, manganese, molybdenum (for N-fixation), and zinc. Some of these are toxic at levels above those required for optimum plant growth. Most of the micronutrients are required for adequate function of essential enzymes. Photosynthetic processes use manganese, iron, chlorine, and zinc. Since the micronutrients are required at such low levels, soil normally provides sufficient amounts.


