11.8: Have You Thanked a Clod Today?
- Page ID
- 285362
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)A common bumper sticker is one that asks the question, “Have you thanked a green plant today?” this is an obvious reference to plants whose photosynthesis produces the food that we and most other animals depend upon for our existence. An even more fundamental question is whether we have thanked the soil — the clods of dirt — upon which green plants depend for their existence. Good, productive soil combined with a suitable climate and adequate water is the most valuable asset that a nation can have. Vast areas of the world lack this fundamental asset, and the people living in areas with poor soil often suffer poverty and malnutrition as a result. Furthermore, areas that once had adequate soil have seen it abused and degraded to the extent that it is no longer productive. One of the central challenges faced by the practice of green chemistry and industrial ecology is to retain and enhance the productive qualities of soil.
The remainder of this chapter addresses soil and those aspects of agriculture related specifically to soil. The biological aspects of agriculture and the production of food and biomass are discussed in Chapter 12.
What is Soil?
Soil is a term that actually describes a wide range of finely divided mineral matter containing various levels of organic matter and water that can sustain and nourish the root systems of plants growing on it. Soil is largely a product of the weathering of rock by physical, chemical, and biochemical processes that produces a medium amenable to the support of plant growth. A healthy soil contains water available to plants, has a somewhat loose structure with air spaces, and supports an active population of soil-dwelling organisms, including fungi and bacteria that degrade dead plant biomass and animals, such as earthworms. Although the solids in a typical soil are composed of about 95% inorganic matter, some soils contain up to 95% organic matter, and some sandy soils may have only about 1% organic matter.
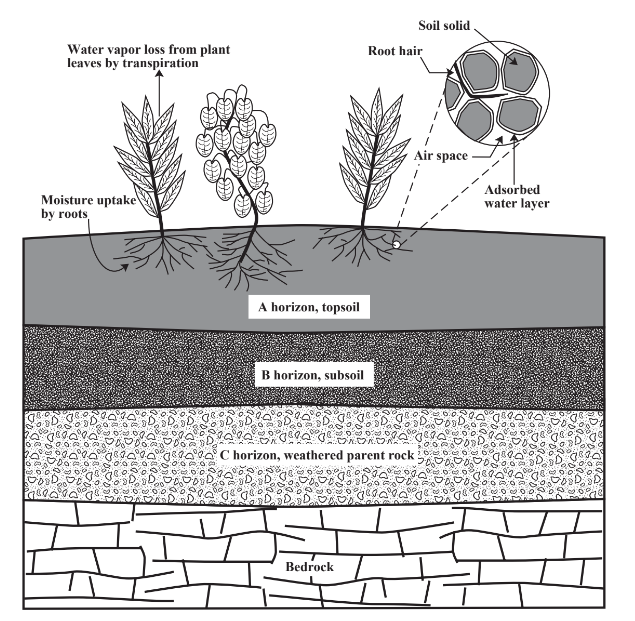
Figure 11.4 shows the major aspects of the physical structure of soil. Soil is divided into layers called horizons formed by weathering of parent rock, chemical processes, biological processes, and the action of water including leaching of colloidal matter to lower horizons. The most important of these for plant growth is topsoil. Plant roots permeate the topsoil and take water and plant nutrients from it. Topsoil is the layer of maximum biological activity. The rhizosphere is the part of topsoil in which plant roots are especially active and in which the elevated levels of biomass are composed of plant roots and microorganisms associated with them. There are strong synergistic relationships between plant root systems and microorganisms in the rhizosphere. The surfaces of root hairs are commonly colonized by microorganisms, which thrive upon carbohydrates, amino acids, and root-growth-lubricant mucigel secreted from the roots.
Inorganic Solids in Soil
Reflecting the fact that the two most common elements in the earth’s crust are oxygen and silicon (see Section 11.2), silicates are the most common mineral constituents of soil. These include finely divided quartz (SiO2), orthoclase (KAlSi3O8), and albite (NaAlSi3O8). Other elements that are relatively abundant in Earth’s crust are aluminum, iron, calcium, sodium, potassium, and magnesium; their abundance is reflected by various minerals such as epidote (4CaO•3(AlFe)2O3•6SiO2•H2O), geothite (FeO(OH)), magnetite (Fe3O4), calcium and magnesium carbonates (CaCO3, CaCO3•MgCO3), and oxides of manganese and titanium in soil. Soil parent rocks undergo weathering processes to produce finely divided colloidal particles, by far the most abundant of which are clays. These secondary minerals hold moisture and mineral nutrients, such as K+ required for plant growth, that are accessible by plant roots and are repositories of plant nutrients. Inorganic soil colloids can absorb toxic substances in soil, thus reducing the toxicity of substances that would harm plants. It is obvious that the abundance and nature of inorganic colloidal material in soil are important factors in determining soil productivity.
Soil Organic Matter
The few percent of soil mass consisting of organic matter has a strong influence upon the physical, chemical, and biological characteristics of soil. Among its important effects in soil organic matter is effective in holding soil moisture and it holds and exchanges with plant roots some of the ions that are required as plant nutrients. Temperature, moisture, and climatic conditions significantly affect the kinds and levels of soil organic matter. Cold, wet conditions in which soil stays saturated with moisture preventing access of microorganisms to oxygen tend to prevent complete biodegradation of plant residues that compose soil organic matter allowing it to accumulate. This is clearly illustrated by accumulation of peat in Ireland and other locales with similar climatic conditions such that most of the solid soil is composed of organic matter. Tropical conditions, especially with alternate wet and dry seasons, can result in loss of soil organic matter. One reason that the soil supporting tropical rain forests degrades so quickly when the trees are removed is that the organic matter in the soil undergoes rapid biodegradation when the forest cover is removed,
The plant biomass residues that form soil organic matter undergo a biodegradation process by the action of soil bacteria and fungi in which the cellulose in the biomass is readily degraded leaving modified residues of the lignin material that binds the cellulose to the plant matter. This is the process of humification and the residue is soil humus, a black organic material of highly varied chemical structure. A fraction of soil humus is soluble in water (see the discussion of humic substances in water, Chapter 9, Section 9.3), especially when base is present in the water. Another fraction called humin does not dissolve and stays in the solid soil.
Though composing usually not more than a few percent of soil, soil humus has a very strong influence on the characteristics of soil. It has a strong affinity for water and holds much of the water in a typical soil. Primarily because of their carboxylic acid (-CO2H) groups, soil humic molecules exchange H+ ion and act to buffer the pH of water in soil (the soil solution). Humic substances bind metal ions and other ionic plant nutrients. Soil humus also binds and immobilizes organic materials, such as herbicides applied to soil.
Water in Soil and The Soil Solution
Water in soil is required for plants. This water is taken up by plant root hairs, transferred through the plant, and evaporated from the leaves, a process called transpiration. The quantities of water involved are enormous; for example, the water transpired to produce a kilogram of dry hay can amount to several hundred kg. Most of the water in normal soils is not present as visible liquid, but is absorbed to various degrees upon the soil solids. In fact, a condition in which all the spaces in soil are filled with water — waterlogging — slows the growth of most plants. The water that is available in soil is called the soil solution and contains a number of dissolved materials, including plant nutrients. It plays an essential role in transferring substances, such as dissolved metal cations, between roots and the soil solid. Cations commonly present in the soil solution include H+, Ca2+, Mg2+, Na+, and K+ along with very low levels of Fe2+, Mn2+, and Al3+. Commonanions present are HCO3-, CO32-, HSO4-, SO42-, Cl-, and F-.


