9.1: H₂O - Simple Formula, Remarkable Molecule
- Page ID
- 285340
Water composes the hydrosphere, which is described and discussed in Section 8.2. This chapter enlarges upon that discussion and the crucial role of water in the environment. In this chapter and elsewhere in the book the term natural water is used in reference to water that occurs in the environment in comparison to water in the anthrosphere, such as water in municipal water distribution systems. Water has a special place in living organisms and the environment. The quality and availability of water are of the utmost importance to humans and the environment. Although scarce and badly polluted in many parts of the world, water is arguably the most recyclable of the substances that compose Earth’s green capital and it is accurately described as the ultimate green substance.
This chapter addresses major aspects of the sustainability of water. The first of these is water pollution, which degrades water quality and can make it unfit for use or to support life. The second major area addressed is how water quality can be maintained and enhanced, largely through various water treatment processes. A third major area is water pollution prevention and a fourth is recycling of the water resource which is arguably nature’s most recyclable material.
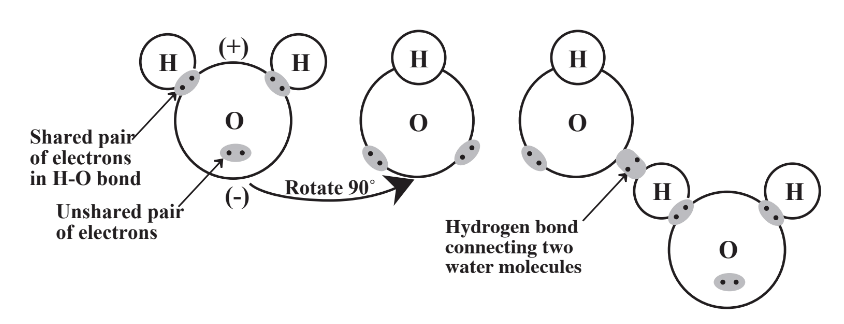
The chemical formula of water, H2O, is probably the best known of all compounds. This simple formula represents a substance that is unique and complex in its behavior. These special properties are due to the molecular structure of the H2O molecule represented in Figure 9.1. There are four pairs of electrons in the outer electron shell of the O atom in the H2O molecule, two of which compose the bonds between the H and O atoms and two of which are lone pairs. The distribution of these pairs as far apart as possible around an imaginary sphere representing the outer electron shell of the O atoms results in the two H-O bonds being located at an angle rather than a straight line. The side of the molecule with the two H atoms has a partial positive charge and the side with the two non-bonding pairs has a partial negative charge, so the molecule is polar. This polarity and the ability of the H atoms on one molecule to form hydrogen bonds to O atoms on other molecules determine the remarkable chemical and physical diversity of water.
Especially because of their hydrogen bonding capability, water molecules are strongly attracted to each other. This means that a large amount of heat energy must be put into a mass of water to enable the molecules to move more rapidly as the temperature is raised. This gives water a very high heat capacity. A very large amount of energy must be put into a mass of ice to break the hydrogen bonds holding the molecules in place in the solid as it melts and an equally large amount of heat energy is released when liquid water freezes. Thus water has a very high latent heat of fusion. Even more energy per unit mass is required to convert liquid water to vapor (steam) and an equal amount of energy is released when water vapor condenses to liquid. This means that water has a very high heat of vaporization.
The ability of water to absorb, release, and store heat is crucial to its role in the environment and its practical uses.1 Water’s high heat capacity stabilizes temperatures of organisms and geographical areas. Steam produced in a boiler can be transferred through insulated pipes to remote locations and condensed to release heat. The heat released when atmospheric water condenses warms the surrounding air and is the driving force behind tropical storms. Europe owes its relatively mild weather despite its northern latitudes to heat carried by water across the North Atlantic Ocean from the Gulf of Mexico. As the water releases heat and cools along the European coasts, its density increases and it flows at lower ocean depths back to the Gulf of Mexico to repeat the cycle. Water’s high latent heat of fusion stabilizes temperatures of bodies of water at water’s freezing point (0 ̊C).
In addition to those listed above, there are other unique and environmentally important qualities of water. It is an excellent solvent, especially for ionic substances, making it important in the transport of nutrients and wastes in the biosphere and in the dissolution, transport, and deposition of minerals in the geosphere. Water has a very high surface tension, a controlling factor in physiology and a property involved in formation of drops in rainfall. The temperature/density relationship of water causes bodies of water to become stratified (see Figure 9.4), a property that strongly affects the chemical behavior of water in stratified bodies of water. The fact that the maximum density of water occurs as a liquid at 4 ̊C means that solid ice floats. If that were not the case, bodies of water in northern climates would become frozen solid with only a surface layer thawing during the summer. Water is largely transparent to visible light, which can penetrate the liquid to some depth and enable photosynthetic phytoplankton and some bottom-rooted plants in shallow water regions to carry out photosynthesis and produce the biomass that is the basis of aquatic food chains (see Section 8.5).


