9.2: Occurrence, Availability , and Utilization of Water on Earth
- Page ID
- 285341
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)Much of Earth’s approximately 1.4 billion cubic kilometers (1.4×109km3) water that is not chemically bound circulates through the hydrologic cycle represented in the illustration of the hydrosphere shown in Figure 8.1. About 97.6% of this water is salt water in oceans. Of the approximately 33 million km3 of water remaining, 86.9% is in the solid form in snowpack, ice and glaciers; 12.0% is accessible water under ground (groundwater), 0.37% is in freshwater lakes, reservoirs, and ponds; 0.31% is in saline lakes; 0.19% is soil moisture; 0.19% is in biospheric organisms; 0.039% is in the atmosphere as vapor and cloud droplets; 0.011% is in wetlands; and only 0.0051% constitutes the water in all Earth’s rivers, streams, and canals. Therefore, a remarkably small percentage of Earth’s water is relatively available for use, a major factor in the sustainability of this crucial part of Earth’s natural capital.
Fresh surface water and groundwater are the major sources for human use. Desalinated seawater is a growing source of fresh water in some areas that do not have access to this valuable resource. Most seawater desalination plants are in the Middle East, an arid region with generally abundant sources of energy that can be used for desalination. The world’s largest desalination plant is the Jebel Ali multi-stage flash distillation unit in the United Arab Emirates that can produce 300 million cubic meters of potable water per year. There are significant resources of brackish (saline) groundwater throughout the world. In many cases this water has a lower salt content than seawater making it easier and more economical to desalinate.
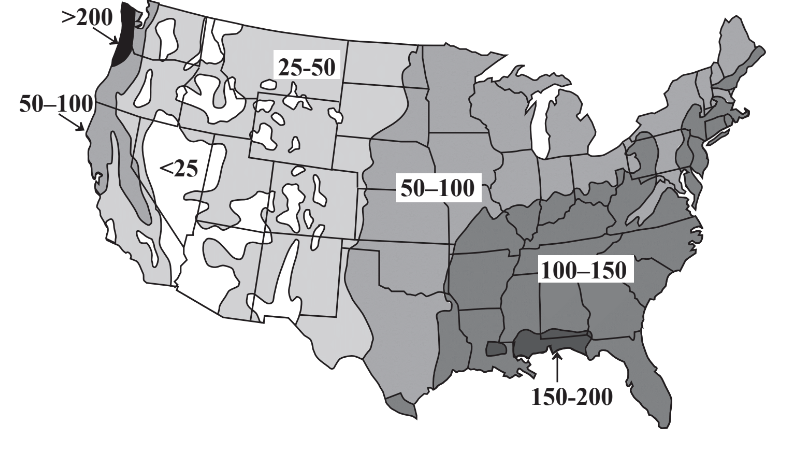
A major concern regarding water’s availability is its uneven distribution with respect to time and location. Some areas experience tremendous rainfall and flooding during wet (monsoon)seasons with dry conditions in between. Whereas these fluctuations are predictable and compensation may be made for them, greater problems occur with long-term droughts. Regions of Africa are periodically afflicted with droughts that last for several years, killing crops and animals and inflicting great hardship on the people in the region. Evidence from tree rings indicates a pre-Columbian drought of almost three centuries duration in what is now the southwestern U.S.! Uneven geographic distribution of water occurs throughout the world and is illustrated for the continental U.S. in Figure 9.2. It is seen that precipitation is generally adequate in the eastern part of the country, although damaging droughts do occur in this region. However, the western continental U.S. has a shortage of precipitation with essentially permanent drought conditions in some regions including Nevada, Arizona, and southern California. The problem is exacerbated by the popularity of these regions as areas in which people want to live and the demands that they put on limited water resources.
Surface Streams of Water
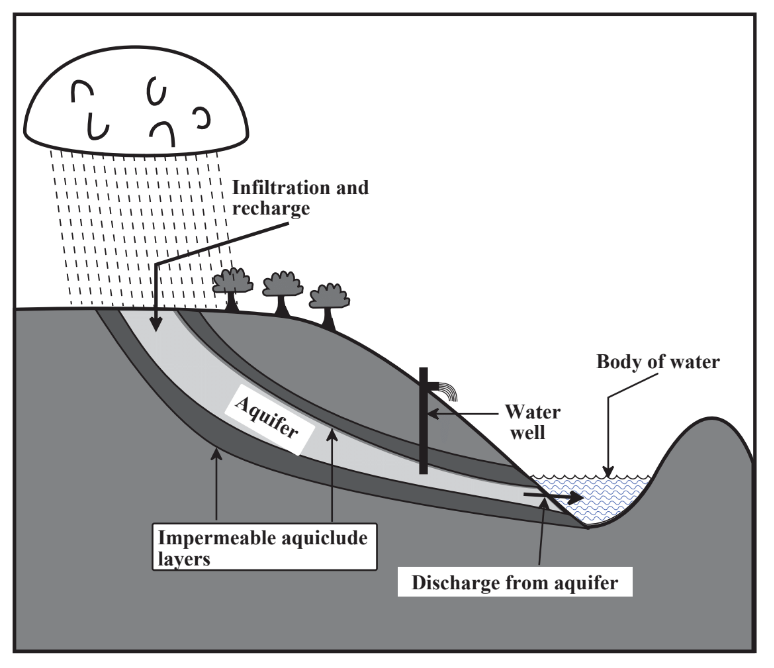
A major source of water is surface water that flows in streams and rivers. The areas of land upon which precipitation falls to provide flows of surface water is called the watershed. Insofar as water utilization is concerned, watersheds constitute one of the most important connections between the geosphere and the hydrosphere; the nature of the watershed largely determines the quantity and quality of water available for human use. A good watershed retains water for a significant length of time, which reduces flooding, allows for a steady flow of runoff water, and maximizes recharge of water into groundwater reservoirs (aquifer recharge, Figure 9.3). Measures employed to enhance watershed quality include minimization of cultivation and forest cutting, especially on steeply sloping sections of watershed, construction of terraces and waterways planted to grass on cultivated land to minimize erosion and accumulation of sediment, construction of small impoundments on feeder streams of the watershed, and of course minimization of pollution such as from herbicides.
An important aspect of streams that affects water utilization and quality is their ability to mobilize sedimentary materials through erosion, transport materials along with stream flow, and deposit them as solids. Normally it is desirable for streams used as a source of water to have minimal sedimentary material, which has to be removed during water treatment.
Most rivers, once free flowing and unimpeded by human intervention, have been modified by humans to generate power, for water supply, and reduce effects of flooding. Beginning with impoundment of water from the Owens Valley in northern California and extending to the Feather, Sacramento, and Colorado rivers, Los Angeles’ voracious thirst for water is served by a vast system of dams, canals, and tunnels. Diversion of water from the Owens valley has turned a formerly productive agricultural area into one of limited agricultural use. Many of the adverse effects of water diversion have resulted from dams built to confine rivers. As a result of dam construction many once beautiful river valleys have been covered with water and productive farmland in river valleys has been lost. For example, the beautiful Hetch-Hetchy Valley in Yosemite National Park in California has been inundated since the early 1900s by construction of a dam built to provide water and hydroelectric power to San Francisco. Serious proposals are now being considered to remove the dam and restore the valley to its former beauty.
Standing Bodies of Water
Much of the water that humans use comes from standing bodies of water including natural lakes and reservoirs constructed by placing dams on rivers. Wetlands are bodies of water shallow enough to support the growth of bottom-rooted plants. Estuaries form where fresh river water flows into oceans. They have unique physical, chemical, and biological properties because of the mixing of fresh water and saltwater. Wetlands and estuaries are important breeding grounds for a number of organisms and it is crucial that they be preserved. Many wetland areas have been drained for agricultural land and an important effort in conservation is their preservation and restoration.
An important characteristic of a lake or reservoir that develops during the summer as a result of the temperature/density behavior of water is stratification, shown in connection with water chemistry in Figure 9.4. Exposed to atmospheric oxygen and light, the top layer, the epilimnion, normally has a significant concentration of dissolved oxygen and is oxic. Photosynthetic algae
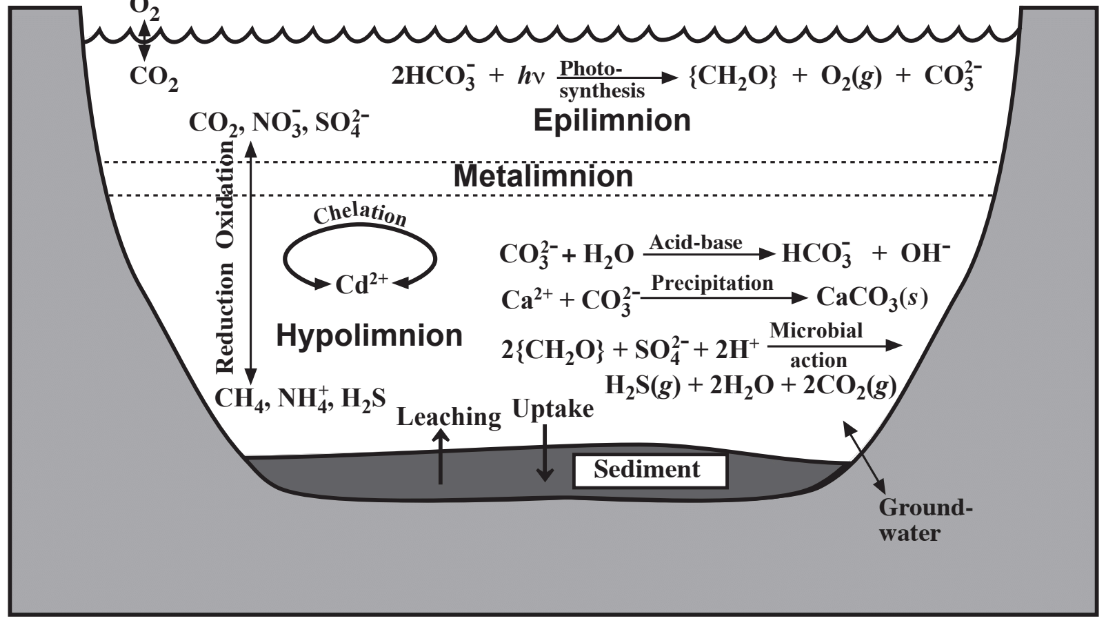
thrive in the epilimnion and during daylight produce O2 by photosynthesis. Isolated from atmospheric oxygen, the hypolimnion located in the bottom regions of the body of water becomes anoxic as O2 is consumed by bacteria. Chemically oxidized species including CO2, NO3-, and SO42- predominate in the epilimnion and chemically reduced species such as CH4, NH4+, and H2S are found in the hypolimnion. During fall in temperate climates, cooling of the epilimnion causes it to become more dense and to sink resulting in overturn of the body of water. This phenomenon tends to stir up sedimentary material and release nutrients from the sediment into the water.
Groundwater
Figure 9.3 illustrates the pathway of groundwater into aquifers from precipitation falling on a watershed and infiltrating the aquifer through a recharge zone. The zone of saturation consists of the geospheric rock and soil layers in which all the pores are filled with water, the top of which defines the water table. The fraction of the aquifer formation consisting of pores is the porosity and the ability of the formation to allow movement of water is its permeability. Generally, high permeability and high porosity are desirable properties of aquifer formations that allow relatively large amounts of water to be withdrawn through water wells drilled into the aquifers. The quality of groundwater is strongly affected by its contact with mineral formations in the geosphere. Infiltration through rock and sand purifies water filtering out microorganisms and suspended solids. Contact with geospheric solids largely determines the chemical composition of water by adding desirable levels of dissolved Ca2+ ion and alkalinity and sometimes undesirable solutes such as sodium chloride.
Artesian Wells
Artesian aquifers are those confined by dense clay or shale such that water flows naturally from an artesian well drilled into them. The name comes from the Roman city of Artesum at the site of the French town of Artois known for the free-flowing water from wells dug in the Middle Ages. Artesian wells are often highly prized sources of water. Some modern day bottling companies have leased or purchased sites of artesian wells and advertise their product as artesian water, although it does not differ in quality from pumped well water. Many artesian wells have lost their free-flowing qualities because of depletion of their water resource.


