7.7: Enzymes
- Page ID
- 285322
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)Recall from Chapter 5, Section 5.5, that catalysts are substances that speed up a chemical reaction without themselves being consumed in the reaction. Catalysis is one of the most important aspects of green chemistry because the ability to make reactions go faster as well as more efficiently, safely, and specifically means that less energy and raw materials are used and less waste is produced. Biochemical catalysts called enzymes include some of the most sophisticated of catalysts. Enzymes speed up biochemical reactions by as much as ten- to a hundred million-fold. They often enable reactions to take place that otherwise would not occur, that is, they tend to be very selective in the reactions they promote. One of the greatest advantages of enzymes as catalysts is that they have evolved to function under the benign conditions under which organisms exist. This optimum temperature range is generally from about the freezing point of water (0 ̊C) to slightly above body temperature (up to about 40 ̊C). Chemical reactions go faster at higher temperatures, so there is considerable interest in enzymes isolated from microorganisms that thrive at temperatures near the boiling point of water (100 ̊C) in hot water pools heated by underground thermal activity such as are found in Yellowstone National Park.
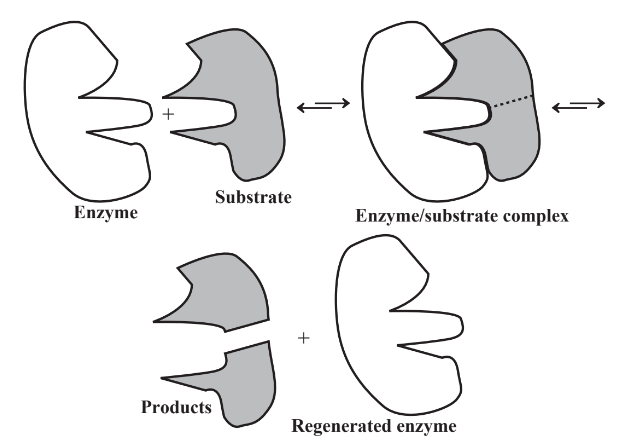
Enzymes are proteinaceous substances. Their structure is highly specific so that they bind with whatever they act upon, a substance called a substrate. The basic mechanism of enzyme action is shown in Figure 7.9. As indicated by the figure, an enzyme recognizes a substrate by its shape, bonds with the substrate to produce an enzyme-substrate complex, causes a change such as splitting a substrate in two with addition of water (hydrolysis), then emerges unchanged to do the same thing again. The basic process can be represented as follows:
\[\textrm{enzyme + substrate} \leftrightarrows \textrm{ enzyme-substrate complex} \leftrightarrows \textrm{ enzyme + product}\]
Note that the arrows in the formula for enzyme reaction point both ways. This means that the reaction is reversible. An enzyme-substrate complex can simply go back to the enzyme and the substrate. The products of an enzymatic reaction can react with the enzyme to form the enzyme-substrate complex again. It, in turn, may again form the enzyme and the substrate. Therefore, the same enzyme may act to cause a reaction to go either way.
In order for some enzymes to work, they must first be attached to coenzymes. Coenzymes normally are not protein materials. Some of the vitamins are important coenzymes.
The names of enzymes are based upon what they do and where they occur. For example, gastric protease, commonly called pepsin,is an enzyme released by the stomach (gastric), which splits protein molecules as part of the digestion process (protease). Similarly, the enzyme produced by the pancreas that breaks down fats (lipids) is called pancreatic lipase. Its common name is steapsin. In general, lipase enzymes cause lipid triglycerides to dissociate and form glycerol and fatty acids.
Lipase and protease enzymes are hydrolyzing enzymes, which enable the breakdown of high-molecular-mass biological compounds and add water, one of the most important types of the reactions involved in digestion of food carbohydrates, proteins, and fats. Recall that the higher carbohydrates humans eat are largely disaccharides (sucrose, or table sugar) and polysaccharides(starch). These are formed by the joining together of units of simple sugars, C6H12O6, with the elimination of an H2O molecule at the linkage where they join. Proteins are formed by the condensation of amino acids, again with the elimination of a water molecule at each linkage. Fats are esters which are produced when glycerol and fatty acids link together. A water molecule is lost for each of these linkages when a protein, fat, or carbohydrate is synthesized. In order for these substances to be used as a food source, the reverse process must be catalyzed by hydrolyzing enzymes to break down large, complicated molecules of protein, fat, or carbohydrate to simple, soluble substances which can penetrate a cell membrane and take part in chemical processes in the cell.
An important biochemical process is the shortening of carbon atom chains, such as those in fatty acids, commonly by the elimination of CO2 from carboxylic acids. For example, pyruvate decarboxylase enzyme removes CO2 from pyruvic acid,
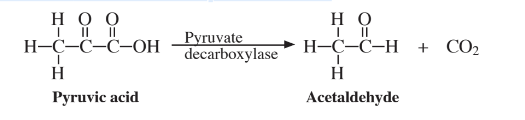
to produce a compound with one less carbon. It is by such carbon-by-carbon breakdown reactions that long chain compounds are eventually degraded to CO2 in the body. Another important consequence of this kind of reaction is the biodegradation of long-chain hydrocarbons by the action of microorganisms in the water and soil environments.
Energy is exchanged in living systems largely by oxidation and reduction mediated by oxidoreductase enzymes. Cellular respiration is an oxidation reaction in which a carbohydrate, C6H12O6, is broken down to carbon dioxide and water with the release of energy
\[\ce{C6H12O6 + 6O2 \rightarrow 6 CO2 + 6H2O + energy}\]
Actually, such an overall reaction occurs in living systems by a complicated series of individual steps including oxidation. The enzymes that bring about oxidation in the presence of free O2 are called oxidases.
In addition to the major types of enzymes discussed above there are numerous other enzymes that perform various functions. Isomerases form isomers of particular compounds. For example, isomerases convert several simple sugars with the formula C6H12O6 to glucose, the only sugar that can be used directly for cell processes. Transferase enzymes move chemical groups from one molecule to another, lyase enzymes remove chemical groups without hydrolysis and participate in the formation of C=C bonds or addition of species to such bonds, and ligase enzymes work in conjunction with ATP (adenosine triphosphate, a high-energy molecule that plays a crucial role in energy-yielding, glucose-oxidizing metabolic processes) to link molecules together with the formation of bonds such as carbon-carbon or carbon-sulfur bonds.
Enzymes are affected by the conditions and media in which they operate. Among these is the hydrogen ion concentration (pH). An interesting example is gastric protease which requires the acid environment of the stomach to work well but stops working when it passes into the much more alkaline medium of the small intestine. This prevents damage to the intestine walls, which would occur if the enzyme tried to digest them. Part of the damage to the esophagus from reflux esophagitis (acid reflux) is due to the action of gastric protease enzyme that flows back into the esophagus from the stomach with the acidic stomach juices. Temperature is critical for enzyme function. Not surprisingly, the enzymes in the human body work best at around 37 ̊C (98.6 ̊F), which is the normal body temperature. Heating these enzymes to around 60 ̊C permanently destroys them. Some bacteria that thrive in hot springs have enzymes that work best at temperatures as high as that of boiling water. Other “cold-seeking” bacteria have enzymes adapted to near the freezing point of water.
Immobilized Enzymes in Green Chemistry
As noted above, enzymes in organisms have a variety of existing and potential uses in the practice of green chemistry. In many cases it is advantageous to isolate the enzyme used for a particular process from cells and use it outside the cellular environment. In a batch synthesis this can be done by mixing the enzyme with the reactants and allow it to catalyze the desired reaction making sure that optimum conditions of temperature and pH are maintained. This approach has several disadvantages. Enzymes are expensive and isolation of the enzyme from the reaction mixture is usually very costly and often not possible. Enzyme contamination of the product can cause difficulties.
The solution to the problem outlined above is often to employ enzyme immobilization that uses a two-phase system in which the enzyme is in one phase and the reaction occurs in another. Sequestration of the enzyme in a separate phase enables its re-use or continuous use in flow-through systems and prevents enzyme contamination of the products. Several major techniques have been employed for enzyme immobilization including (1) adsorption onto a solid, (2) covalent binding onto a separate phase, (3) entrapment in a separate phase, (4) confinement with a membrane that allows transport of reactants and products but retains the enzyme. Ideally the matrix holding the enzyme should be capable of holding the enzyme as well as being inert, physically strong, chemically stable, and capable of being regenerated. The most common materials used to hold enzymes have been porous carbon, ion-exchange matrices, clays, polymeric resins, hydrous metal oxides, and glasses.
The procedure for immobilization of an enzyme begins with mixing the enzyme and the solid materials under suitable conditions of pH and ionic strength, sometimes along with binding agents. The support holding the immobilized enzyme is then incubated for some time. Finally, excess enzyme and, where used, binding agents are washed off of the support.
Rather than isolating enzymes and immobilizing them on a support, living microorganism cells, usually those of bacteria, are often used. Two main categories of immobilization are employed, attachment and entrapment. The simplest kind of immobilization is aggregation cross-linking in which the microbial cells form networks that compose their own support. This approach is usually confined to batch processes. Otherwise the major kinds of attachment immobilization are covalent binding, binding on ion-exchangers, adsorption binding, and biofilm formation. Probably the most common form of biofilm reactor is the trickling filter used for wastewater treatment (see Chapter 9) in which growing bacteria and protozoa form a film over solid support material (usually rock) and the wastewater is sprayed over the biofilm. This enables contact of the immobilized microorganisms with both the biodegradable material in the wastewater and atmospheric oxygen. Entrapment of microorganisms may be on organic polymer, on inorganic polymer, or behind a semi-permeable membrane.
Use of living organisms as sources of immobilized enzymes offers the advantage of not having to isolate the enzyme and, in cases where the organism is reproducing, of continuously replenishing the enzyme. A disadvantage includes having to maintain conditions under which the organism is viable. Also, living cells harbor numerous enzymes so side-reactions and unwanted products can be a problem.
Effects of Toxic Substances on Enzymes
Toxic substances may destroy enzymes or alter them so that they function improperly or not at all. Among the many toxic substances that act adversely with enzymes are heavy metals, cyanide, and various organic compounds such as insecticidal parathion. Many enzyme active sites through which an enzyme recognizes and bonds with a substrate contain -SH groups. Toxic heavy metal ions such as Pb2+ or Hg2+ are “sulfur seekers” that bind to the sulfur in the enzyme active site causing the enzyme to not function. A particularly potent class of toxic substances consists of the organophosphate “nerve gases” such as Sarin that inhibit the acetylcholinesterase enzyme required to stop nerve impulses. Very small doses of Sarin stop respiration by binding with acetylcholinesterase and causing it to not work. Discussed further in Section 7.9 under the topic of toxicological chemistry, toxicity to enzymes is a major consideration in the practice of green chemistry.


