4.7: Predicting Formulas of Covalently Bound Compounds
- Page ID
- 285258
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
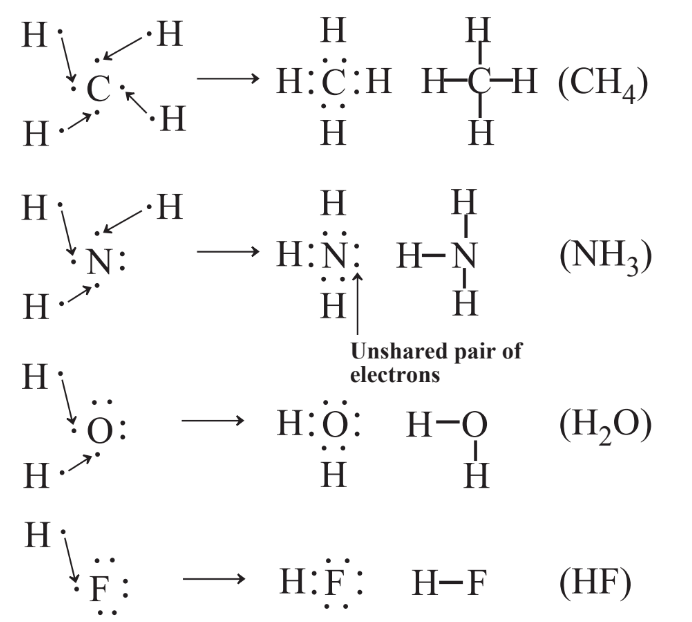
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)It is often possible to predict the formulas of molecules using the Lewis symbols of the elements in the compound with the octet rule for chemical bonding. This is shown in Figure 4.8 for the hydrogen compounds of several elements in the second period of the periodic table.
The prediction of chemical bonds in compounds in which H is bonded to another atom is very simple because each H atom has to be involved in sharing two electrons and the other kind of atom normally has to have a total of 8 electrons in its valence shell octet; these may be shared in bonds or present as unshared pairs. As an example, consider a well-known compound of carbon, carbon dioxide, chemical formula CO2. The Lewis symbol of C and those of the two O atoms can be used to deduce the Lewis formula of CO2 as shown in Figure 4.9.
As another example of the application of the octet rule, consider hydrogen peroxide, H2O2. This compound’s formula looks a lot like that of water, but it is a lot different from water. Hydrogen peroxide decomposes to release oxygen:
\[\ce{2H2O2 (liquid) \rightarrow O2(gas) + 2H2O(liquid)}\]
As a liquid in the form of a concentrated aqueous solution, hydrogen peroxide provides a source of oxygen so potent that it has been used in rockets. It was the treacherous oxidant used along with hydrazine (N2H4) fuel in the German Luftwaffe’s Messerschmidt 163 rocket plane at the end of World War II. Trailing an exhaust of lethal NO2 gas, this minuscule manned missile (on the rare occasions when it worked according to plan) was propelled rapidly into the lower stratosphere, then glided down through waves of Allied bombers, attempting to nick them with machine gunfire as it plummeted back to Earth. Few bombers were damaged but many Me-163 pilots died in the attempt, some as the result of explosions, fires, and spills from the hydrogen peroxide oxidant. Hydrogen peroxide decomposed over a catalyst was also used as a source of oxygen for the diesel engines on several German submarines near the end of World War II. Pre-dating nuclear submarines, these potentially deadly craft were the first true submersibles.
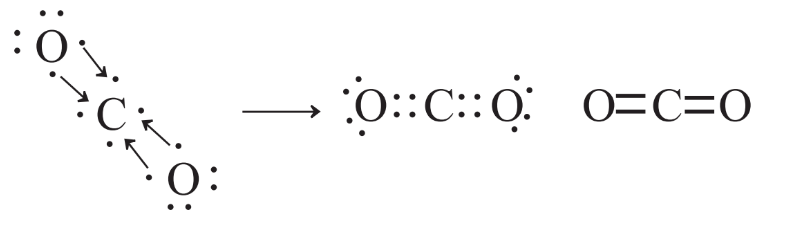
In assembling the structure of the hydrogen peroxide molecule, one has simply to work with two O atoms each contributing 6 valence electrons and two H atoms each with 1 valence electron. The Lewis formula of the H2O2 molecule is

showing that all of the 14 total valence electrons are involved in chemical bonds and both oxygens have octets of outer-shell electrons.
Despite the evil nature of concentrated solutions of hydrogen peroxide, it can be regarded as a green compound in more dilute solutions, such as the 3% hydrogen peroxide commonly used to kill bacteria in treating wounds. Among its green applications, dilute hydrogen peroxide makes an effective and safe bleaching agent that is much safer to handle than elemental chlorine commonly used for bleaching and that does not produce the potentially toxic byproducts that chlorine generates. And even though it kills bacteria, hydrogen peroxide can be pumped underground to serve as an oxidant for acclimated bacteria that attack wastes that have been placed in or seeped into underground locations
Molecules That Do Not Obey the Octet Rule
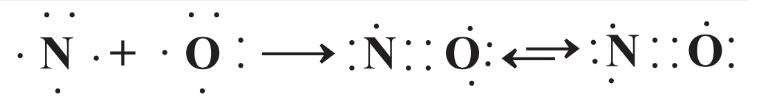
In some cases the octet rule is not obeyed. This occurs when a molecule has an uneven number of electrons so that it is impossible for each atom to have an octet (an even number) of electrons. A simple example of this is nitric oxide, NO, made from an atom of N with 5 valence electrons and one of O with 6 valence electrons. The resulting molecule is shown in Figure 4.10. Since the uneven number of 11 valence electrons cannot provide complete octets of electrons around both the N and O atoms simultaneously, the NO molecule is shown in two forms in which one atom has 8 valence electrons and the other has 7. These are known as resonance structures.
Unequal Sharing of Electrons
The Lewis formula for water,
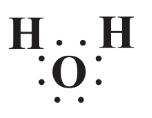
indicates that the molecule is not symmetrical and the two H atoms are located on one side of the molecule and the O atom on the other side. One might think that the electrons shared between Hand O are shared equally. But such is not the case because the relatively larger O atom nucleus with its 8 protons has a stronger attraction for the electrons than do the two H atom nuclei, each with only 1 proton. So the shared electrons spend relatively more time around the O atom and less around the H atoms. This gives each H atom a partial positive charge and the O atom a partial negative charge. An unequal distribution of charge such as that makes a body polar and the O-H bonds are polar covalent bonds. Because of this phenomenon, the whole water molecule is polar and can be represented as the following, where the small spheres stand for H atoms and the large one for the O atom:
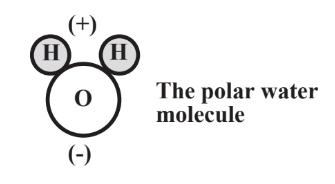
The polar nature of the water molecule has a lot to do with water as a solvent and how it behaves in the environment and in living systems. These aspects are discussed in more detail in Chapter 8.
When Only One Atom Contributes to a Covalent Bond
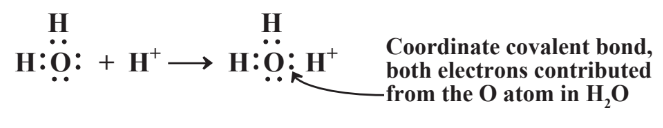
In some cases only one of the two atoms joined by a covalent bond contributes both the electrons in the bond. This occurs with ammonia, NH3, dissolved in water. Water contains dissolved H+ cations, and the more acidic water is, the higher the concentration of H+. The H+ cation, would be stabilized by two electrons which it can get by binding with dissolved NH3 as shown in Reaction 4.7.2. Both of the electrons shared between N and the H+ cation now bound to it as part of a new species, the ammonium ion, NH4+, were contributed by the N atom. Such a covalent bond is called a coordinate covalent bond or a dative bond. In the case of NH4+, once the coordinate covalent N-H bond is formed, it is indistinguishable from all the other N-H bonds.
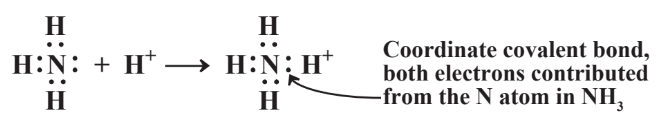
The formation of the coordinate covalent bond in NH4+ is very useful when soil is fertilized with nitrogen. The most economical way to apply nitrogen fertilizer is by injecting NH3 into the soil, but NH3 is a gas that would be expected to rapidly evaporate from soil. Instead, it becomes attached to H+ ion from the water in the soil and is bound to the soil as the NH4+ ion.
Another important example of a coordinate covalent bond occurs in water. As discussed in Section 4.9, acids, which are very important materials commonly dissolved in water, produce the hydrogen ion, H+, in water. This ion does not exist simply dispersed in water. Instead, it binds strongly to a water molecule to produce the hydronium ion, H3O+: