4.1: Chemical Bonds and Compound Formation
- Page ID
- 284484
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)Chemical compounds consist of molecules or aggregates of ions composed of two or more elements held together by chemical bonds. Several examples of chemical compounds including water (H2O), ammonia (NH3), and sodium chloride (NaCl) were given in earlier chapters. This chapter addresses chemical compounds in more detail, including aspects of their green chemistry.
A crucial aspect of chemical compounds consists of the kinds of bonds that hold them together. As noted earlier, these may be covalent bonds composed of shared electrons or ionic bonds consisting of positively charged cations and negatively charged anions. The strengths of these bonds vary and are important in determining compound behavior. For example, chlorofluorocarbons, such as dichlorodifluoromethane, Cl2CF2, are so stable that they persist in the atmosphere and do not break down until reaching very high altitudes in the stratosphere, where the release of chlorine atoms destroys stratospheric ozone. The extreme stabilities of the chlorofluorocarbons are due to the very high strengths of the C-Cl and C-F bonds by which chlorine and fluorine are bonded to a central carbon atom. The proper practice of green chemistry requires that substances that get released to the environment break down readily. Since Cl2CF2 is so stable when released to the atmosphere, it cannot be regarded as being a very good green chemical.
Another important aspect of the way in which chemical compounds are put together is molecular structure, which refers to the shape of molecules. Consider the Cl2CF2 compound just mentioned in which the Cl and F atoms are bonded to a single carbon atom. To represent this molecule as the flat structure (below)

is not totally correct because not all of the 5 atoms in the compound lie in the same plane. Instead, the F and Cl atoms can be visualized as being distributed as far apart as possible in three dimensions around a sphere, at the center of which is the C atom. This can be represented as
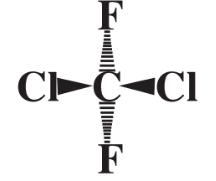
where the two Cl atoms are visualized as being above the plane of the book page toward the reader and the two F atoms are represented as being below the plane of the page away from the reader. The shape of molecules is very important in determining the ways in which they interact with other molecules. For example, the molecules of enzymes that enable metabolism to occur in living organisms recognize the substrate molecules upon which they act by their complementary shapes.
What Are Green Chemical Compounds?
Chemical compounds vary markedly in the degree to which they are “green.”Dichlorodifluoromethane, Cl2CF2, the chlorofluorocarbon discussed above, is definitely not green. That is not because it is toxic — it is one of the least toxic synthetic compounds known — but because it is so extremely stable and persistent in the atmosphere and can cause stratospheric ozone destruction. The compounds that have replaced it, the hydrofluorocarbons and hydrochlorofluorocarbons, are much more green because they do not last long when released in the atmosphere or do not contain ozone-damaging chlorine.
There are several characteristics of compounds that meet the criteria of being green. These are the following:
• Preparation from renewable or readily available resources by environmentally friendly processes
• Low tendency to undergo sudden, violent, unpredictable reactions such as explosions that may cause damage, injure personnel, or cause release of chemicals and byproducts to the environment.
• Nonflammable or poorly flammable
• Low toxicity
• Absence of toxic or environmentally dangerous constituents, particularly heavymetals
• Facile degradability, especially biodegradability, in the environment.
• Low tendency to undergo bioaccumulation in food chains in the environment
An example of a green compound is sodium stearate, common hand soap. This common substance is prepared by reacting byproduct animal fat with sodium hydroxide, which is prepared by passing an electrical current through saltwater. Flushed down the drain, sodium stearate reacts with calcium in water to form a solid, calcium stearate, the white solid that composes “bathtub ring.” This removes the soap from the water and the nontoxic calcium stearate readily undergoes biodegradation so that it does not persist in the environment.


