2.3: What is Environmental Chemistry?
- Page ID
- 284412
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
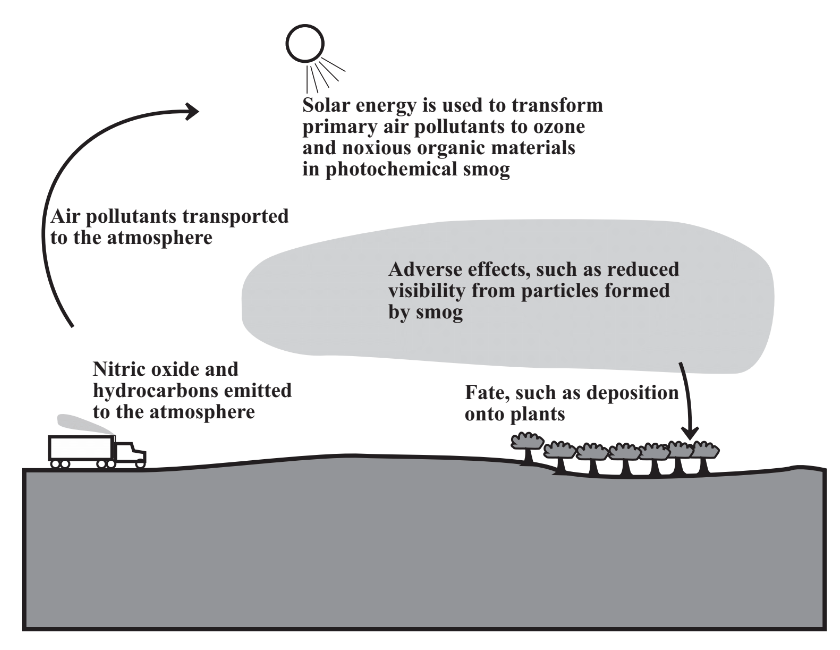
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)The practice of green chemistry must be based upon environmental chemistry. This important branch of chemical science is defined as the study of the sources, reactions, transport, effects, and fates of chemical species in water, soil, air, and living environments and the effects of technology thereon.1 Figure 2.2 illustrates this definition of environmental chemistry with an important type of environmental chemical species. In this example, two of the ingredients required for the formation of photochemical smog — nitric oxide and hydrocarbons — are emitted to the atmosphere from vehicles and transported through the atmosphere by wind and air currents. In the atmosphere, energy from sunlight brings about photochemical reactions that convert nitric oxide and hydrocarbons to ozone, noxious organic compounds, and particulate matter, all characteristic of photochemical smog. Various harmful effects are manifested, such as visibility-obscuring particles in the atmosphere, or ozone, which is unhealthy when inhaled by humans, or toxic to plants. Finally, the smog products end up on soil, deposited on plant surfaces, or in bodies of water.
Figure 2.2.1 showing the five environmental spheres may provide an idea of the complexity of environmental chemistry as a discipline. Enormous quantities of materials and energy are continually exchanged among the five environmental spheres. In addition to variable flows of materials, there are variations in temperature, intensity of solar radiation, mixing, and other factors, all of which strongly influence chemical conditions and behavior.
Throughout this book the role of environmental chemistry in the practice of green chemistry is emphasized. Green chemistry is practiced to minimize the impact of chemicals and chemical processes upon humans, other living organisms, and the environment as a whole. It is only within the framework of a knowledge of environmental chemistry that green chemistry can be successfully practiced.
There are several highly interconnected and overlapping categories of environmental chemistry. Aquatic chemistry deals with chemical phenomena and processes in water. Aquatic chemical processes are very strongly influenced by microorganisms in the water, so there is a strong connection between the hydrosphere and biosphere insofar as such processes are concerned. Aquatic chemical processes occur largely in “natural waters” consisting of water in oceans, bodies of fresh water, streams, and underground aquifers. These are places in which the hydrosphere can interact with the geosphere, biosphere, and atmosphere and is often subjected to anthrospheric influences. Aspects of aquatic chemistry are considered in various parts of this book and are addressed specifically in Chapter 9, “Water, the Ultimate Green Substance.”
Atmospheric chemistry is the branch of environmental chemistry that considers chemical phenomena in the atmosphere. Two things that make this chemistry unique are the extreme dilution of important atmospheric chemicals and the influence of photochemistry. Photochemistry occurs when molecules absorb photons of high-energy visible light or ultraviolet radiation, become energized (“excited”), and undergo reactions that lead to a variety of products, such as photochemical smog. In addition to reactions that occur in the gas phase, many important atmospheric chemical phenomena take place on the surfaces of very small solid particles suspended in the atmosphere and in droplets of liquid in the atmosphere. Although no significant atmospheric chemical reactions are mediated by organisms in the atmosphere, microorganisms play a strong role in determining species that get into the atmosphere. As examples, bacteria growing in the absence of oxygen, such as in cows’ stomachs and under water in rice paddies, are the single greatest source of hydrocarbon in the atmosphere because of the large amounts of methane that they emit. The greatest source of organic sulfur compounds in the atmosphere consists of microorganisms in the oceans that emit dimethyl sulfide. Atmospheric chemistry is addressed specifically in Chapter 10, “Blue Skies for a Green Environment.”
Chemical processes that occur in the geosphere involving minerals and their interactions with water, air, and living organisms are addressed by the topic of geochemistry. A special branch of geochemistry, soil chemistry, deals with the chemical and biochemical processes that occur in soil. Aspects of geochemistry are explained in Chapter 11, “The Geosphere and a Green Earth,” and soil and agricultural chemistry are covered in Chapter 12, “The Biosphere and Feeding a Hungry World.
Environmental biochemistry addresses biologically mediated processes that occur in the environment. Such processes include, as examples, the biodegradation of organic waste materials in soil or water and processes within biogeochemical cycles, such as denitrification, which returns chemically bound nitrogen to the atmosphere as nitrogen gas. The basics of biochemistry are presented in Chapter 7, “The Chemistry of Life and Green Chemistry,” and other aspects of biochemistry are presented in Chapter 12, “The Biosphere and Feeding a Hungry World.” Chapter 14, “Feeding the Anthrosphere: Utilizing Renewable and Biological Materials,” discusses how chemical processes carried out by organisms can produce material feedstocks needed for the practice of green chemistry. The toxic effects of chemicals are of utmost concern to chemists and the public. Chapter 16, “Terrorism, Toxicity, And Vulnerability: Green Chemistry and Technology in Defense of Human Welfare,” deals with aspects of these toxic effects and discusses toxicological chemistry.
Although there is not a formally recognized area of chemistry known as “anthrospheric chemistry,” most of chemical science and engineering developed to date deals with chemistry carried out in the anthrosphere. Included is industrial chemistry, which is very closely tied to the practice of green chemistry. A good way to view “anthrospheric chemistry” from a green chemistry perspective is within the context of industrial ecology. Industrial ecology considers industrial systems in a manner analogous to natural ecosystems. In a system of industrial ecology, various manufacturing and processing operations carry out “industrial metabolism” on materials.A successful industrial ecosystem is well balanced and diverse, with various enterprises that generate products for each other and use each other’s products and potential wastes. A well-functioning industrial ecosystem recycles materials to the maximum extent possible and produces little — ideally no — wastes. Therefore, a good industrial ecosystem is a green chemical system. Industrial ecology and anthrospheric environmental chemistry are addressed in Chapter 13, “TheAnthrosphere, Green Chemistry, and Industrial Ecology."


