1.1: Composition and Structure of the Earth
- Page ID
- 98747
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)The earth has been in a state of continual change since its formation. The major part of this change, involving volcanism and tectonics, has been driven by heat produced from the decay of radioactive elements within the earth. The other source of change has been solar energy, which acts as the driving force of weathering and is the ultimate source of energy for living organisms.
The solar system was probably formed about 4.6 billion years ago, and the oldest known rocks have an age of 3.8 billion years. There is thus a gap of 0.8 billion years for which there is no direct evidence. It is known that the earth was subjected to extensive bombardment earlier in its history; recent computer simulations suggest that the moon could have resulted from an especially massive collision with another body. Although these major collisions have diminished in magnitude as the matter in the solar system has become more consolidated, they continue to occur, with the most recent one being responsible for the annihilation of the dinosaurs and much of the other life on Earth. The lack of many overt signs of these collisions (such as craters, for example) testifies to the dynamic processes at work on the Earth’s surface and beneath it.
Chemical Composition of the Earth
The earth is composed of 90 chemical elements, of which 81 have at least one stable isotope. The unstable elements are 43Tc and 61Pm, and all elements heavier than 83Bi.
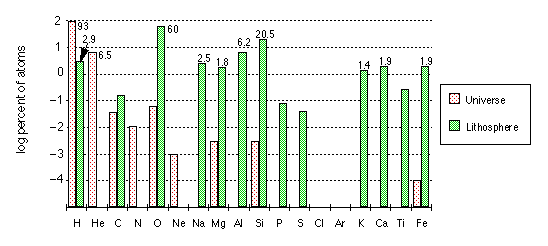
Note that the vertical axis is logarithmic, which has the effect of greatly reducing the visual impression of the differences between the various elements.
The chart gives the abundances of the elements present in the solar system, in the earth as a whole, and in the various geospheres. Of particular interest are the differences between the terrestrial and cosmic abundances, which are especially notable in the cases of the lighter elements (H, C, N) and the noble gas elements (He, Ne, Ar, Xe, Kr).
Example \(\PageIndex{1}\)
Given the mix of elements that are present in the earth, how might they combine so as to produce the chemical composition we now observe?
Solution
Given the mix of elements that are present in the earth, how might they combine so as to produce the chemical composition we now observe? Thermodynamics allows us to predict the composition that any isolated system will eventually reach at a given temperature and pressure. Of course the earth is not an isolated system, although most parts of it can be considered approximately so in many respects, on time scales sufficient to make thermodynamic predictions reasonably meaningful. The equilibrium states predicted by thermodynamics differ markedly from the observed compositions. The atmosphere, for example, contains 0.03% \(\ce{CO2}\), 78% \(\ce{N2}\) and 21% \(\ce{O2}\); in a world at equilibrium the air would be 99% \(\ce{CO2}\).
Similarly, the oceans, containing about 3.5% NaCl, would have a salt content of 35% if they were in equilibrium with the atmosphere and the lithosphere. Trying to understand the mechanisms that maintain these non-equilibrium states is an important part of contemporary environmental geochemistry.
Structure of the Earth
Studies based on the reflection and refraction of the acoustic waves resulting from earthquakes show that the interior of the earth consists of four distinct regions. A combination of physical and chemical processes led to the differentiation of the earth into these major parts. This is believed to have occurred approximately 4 billion years ago.
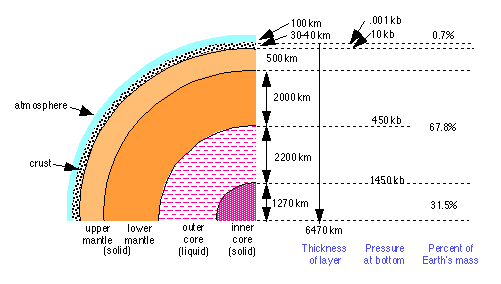
The Earth's Core
The Earth’s core is believed to consist of two regions. The inner core is solid, while the outer core is liquid. This phase difference probably reflects a difference in pressure and composition, rather than one of temperature. Density estimates obtained from seismological studies indicate that the core is metallic, and mainly iron, with 8-10 percent of lighter elements.
Hypotheses about the nature of the core must be consistent with the the core’s role as the source of the earth’s magnetic field. This field arises from convective motion of the electrically conductive liquid comprising the outer core. Whether this convection is driven by differences in temperature or composition is not certain. The estimated abundance of radioactive isotopes (mainly U238 and K40 in the core is sufficient to provide the thermal energy required to drive the convective dynamo. Laboratory experiments on the high-pressure behavior of iron oxides and sulfides indicate that these substances are probably metallic in nature, and hence conductive, at the temperatures (4000-5000K) and pressures (1.3-3.5 million atm) that are estimated for the core. Their presence in the core, alloyed with the iron, would be consistent with the observed density, and would also resolve the apparent lack of sulfur in the earth, compared to its primordial abundance.
The mantle
The region extending from the outer part of the core to the crust of the earth is known as the mantle. The mantle is composed of oxides and silicates, i.e., of rock. It was once believed that this rock was molten, and served as a source of volcanic magma. It is now known on the basis of seismological evidence that the mantle is not in the liquid state. Laboratory experiments have shown, however, that when rock is subjected to the high temperatures and pressures believed to exist in the mantle, it can be deformed and flows very much like a liquid.
The upper part of the mantle consists of a region of convective cells whose motion is driven by the heat due to decay of radioactive potassium, thorium, and uranium, which were selectively incorporated in the crystal lattices of the lower-density minerals that form the mantle. There are several independent sources of evidence of this motion. First, there are gravitational anomalies; the force of gravity, measured by changes in elevation in the sea surface, is different over upward and downward moving regions, and has permitted the mapping of some of the convective cells. Secondly, numerous isotopic ratio studies have traced the exchange of material between oceanic sediments, upper mantle rock, and back into the continental crust, which forms from melting of the upper mantle. Thirdly, the composition of the basalt formed by upper mantle melting is quite uniform everywhere, suggesting complete mixing of diverse materials incorporated into the mantle over periods of 100 million years.
High-pressure studies in the laboratory have revealed that olivine, a highly abundant substance in the mantle composed of Fe, Mg, Si, and O (and also the principal constituent of meteorites) can undergo a reversible phase change between two forms differing in density. Estimates of conditions within the upper mantle suggest that the this phase change could occur within this region in such as way as to contribute to convection. The most apparent effect of mantle convection is the motion it imparts to the earth’s crust, as evidenced by the the external topography of the earth.
The crust
The outermost part of the earth, known also as the lithosphere, is broken up into plates that are supported by the underlying mantle, and are moved by the convective cells within the mantle at a rate of a few centimetres per year. New crust is formed where plates move away from each other under the oceans, and old crust is recycled back into the mantle as where plates moving in opposite directions collide.
The oceanic crust
The parts of the crust that contain the world’s oceans are very different from the parts that form the continents. The continental crust is 10-70 km thick, while oceanic crust averages only 5-7 km in thickness. Oceanic crust is more dense (3.0-3.1 g cm–3) and therefore “floats” on the mantle at a greater depth than does continental crust (density 2.7-2.8 ). Finally, oceanic crust is much younger; the oldest oceanic crust is about 200 million years old, while the most ancient continental rocks were formed 3.8 billion years ago.
|
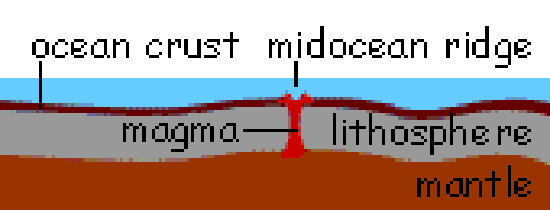
New crust is formed from molten material in the upper mantle at the divergent boundaries that exist at undersea ridges. The melting is due to the rise in temperature associated with the nearly adiabatic decompression of the upper 50-70 km of mantle material as separation of the plates reduces the pressure below. The molten material collects in a magma pocket which is gradually exuded in undersea lava flows. The solidified lava is transformed into crust by the effects of heat and the action of seawater which selectively dissolves the more soluble components. |
||
 |
||
|
An animated view of seafloor spreading. |
Plate collisions
Where two plates collide, one generally plunges under the other and returns to the mantle in a process known as subduction. Since the continental plates have a lower density, they tend to float above the oceanic plates and resist subduction. At continental boundaries such as that of the North American west coast where an oceanic plate pushes under the continental crust, oceanic sediments may be sheared off, resulting in a low coastal mountain range (see here for a nice animation of this process.) Also, the injection of water into the subducting material lowers its melting point, resulting in the formation of shallow magma pockets and volcanic activity. Divergent plate boundaries can cross continents, however; temporary divergences create rift valleys such as the Rhine and Rio Grande, while permanent ones eventually lead to new oceanic basins.
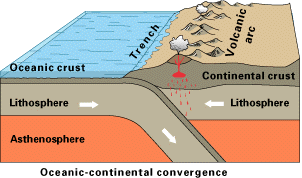
Collision of two continental plates can also occur; the most notable example is the one resulting in the formation of the Himalayan mountain chain.
The Earth is composed of 90 chemical elements, of which 81 have at least one stable isotope. Most of these elements have also been detected in stars. Where did these elements come from? The accepted scenario is that the first major element to condense out of the primordial soup was helium , which still comprises about one-quarter of the mass of the known universe.
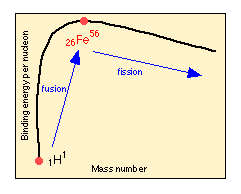
Hydrogen is the least thermodynamically stable of the elements, and at very high temperatures will combine with itself in a reaction known as nuclear fusion to form the next element, 2He4. "Heavier" nuclei (that is , those having high atomic numbers, indicated here by the subscript preceding the element symbol), are more stable than "lighter" ones, so this fusion process can continue up to 56Fe, which is the most energetically stable of all the nuclides. Beyond this point, heavier nuclei slowly become less stable, so fission becomes more likely. FIssion, however, is not considered an important mechanism of primordialnucleosynthesis, so other processes are invoked, as discussed farther below.
Primordial Chemistry
According to the “big bang” theory for which there is now overwhelming evidence, the universe as we know it (that is, all space, time, and matter) had its origin in a point source or singularity that began an explosive expansion about 12-15 billion years ago, and which is still continuing.
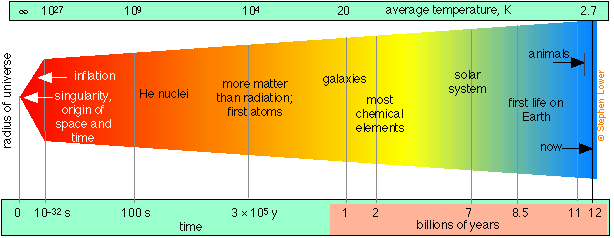
Following a brief period of extremely rapid expansion called inflation, protons and neutrons condensed out of the initial quantum soup after about 10–32 s. Helium and hydrogen became stable during the first few minutes, along with some of the very lightest nuclides up to 7Li, which were formed through various fusion and neutron-absorption processes. Formation of most heavier elements was delayed for about 106 years until nucleosynthesis commenced in the first stars. Hydrogen still accounts for about 93% of the atoms in the universe.
The main lines of observational evidence that support this theory are the 2.7K background radiation that permeates the cosmos (the cooled-down remnants of the initial explosion), and the abundances of the lightest elements. Conventional physics is able to extrapolate back to about the first 10–33 second; what happened before then remains speculative.
Stellar nucleosynthesis
All elements beyond hydrogen were formed in regions where the concentration of matter was large, and the temperature was high; in other words, in stars. The formation of a star begins when the gravitational forces due to a large local concentration of hydrogen bring about a contraction and compression to densities of around 105 g cm–3. This is a highly exothermic process in which the gravitational potential energy is released as heat, about 1200 kJ per gram, raising the temperature to about 107 K. Under these conditions, hydrogen nuclei possess sufficient kinetic energy to overcome their electrostatic repulsion and undergo nuclear fusion:
\[ 4\;_{1}H^{1} \rightarrow _{2}He^{4} +2b^{+}+2g+2n \]
Hydrogen burning
There will be a net mass loss in above process, which will therefore be highly exothermic and is known as “hydrogen burning”. As hydrogen burning proceeds, the helium collects in the core of the star, raising the density to 108 g cm–3 and the temperature to 108 K. This temperature is high enough to initiate helium burning, which proceeds in several steps:
2 2He4 \( \rightarrow \) 4Be8 + g
The first product, 4Be8 has a half life of only 10–16 sec, but a sufficient amount accumulates to drive the following two reactions:
4Be8 + 2He4 \( \rightarrow \) 6C12 + g
6C12 + 1H1 \( \rightarrow \) 7N13 \( \rightarrow \) 6C13 + b+ + g
The size of a star depends on the balance between the kinetic energy of its matter and the gravitational attraction of its mass. As the helium burning runs its course, the temperature drops and the star begins to contract. The course of further nucleosynthesis and the subsequent fate of the star itself depends on the star’s mass.
Small stars
If the mass of the star is no greater than 1.4 times the mass of our sun, the star collapses to a white dwarf, and eventually cools to a dark, dense dead star.
Big stars
In larger stars, the gravitational forces are sufficiently strong to overcome the normal repulsion between atoms, and so gravitational collapse continues. The gravitational energy released in this process produces temperatures of 6  108 K, which are sufficient to initiate a complex series of nuclear reactions known as the carbon-nitrogen cycle. The net reaction of this cycle is the further fusion of hydrogen to helium, in which C12 acts as a catalyst, and various nuclides of nitrogen and oxygen are intermediates. The temperature is sufficiently high, however, to initiate fusion reactions of some of these intermediates:
108 K, which are sufficient to initiate a complex series of nuclear reactions known as the carbon-nitrogen cycle. The net reaction of this cycle is the further fusion of hydrogen to helium, in which C12 acts as a catalyst, and various nuclides of nitrogen and oxygen are intermediates. The temperature is sufficiently high, however, to initiate fusion reactions of some of these intermediates:
6C12 + 6C12 \( \rightarrow \) 10Ne20 + 2He4
2 8O16 \( \rightarrow \) 14Si28 + 2He4
2 8O16 \( \rightarrow \) 16S31 + 0n1
Supernovas
The intense gamma radiation that is produced in some of these reactions breaks some of the product nuclei into smaller fragments, which can then fuse into a variety of heavier species, up to the limit of26Fe56, beyond which fusion is no longer exothermic. The greater relative abundance of elements such as 6C12, 8O16, and 10Ne20 which differ by a 2He4 nucleus, reflects the participation of the latter species in these processes. These exothermic reactions eventually produce temperatures of 8  109 K, while contraction continues until the central core is essentially a ball of neutrons having a radius of about 10 km and a density of 1014 g cm–3. At the same time the outer shell of the star is blasted away in an explosion known as a supernova.
109 K, while contraction continues until the central core is essentially a ball of neutrons having a radius of about 10 km and a density of 1014 g cm–3. At the same time the outer shell of the star is blasted away in an explosion known as a supernova.
Since 26Fe56 has the highest binding energy per nucleon of any nuclide, there are no exothermic processes which can lead to the formation of heavier elements. Fusion into heavier species is also precluded by the electrostatic repulsion of the highly charged nuclei. However, the process of neutron capture can still take place (this is the same process that is used to make synthetic elements). The neutrons are by-products of a large variety of stellar processes, and are present in a wide range of energies. Two general types of neutron capture processes are recognized. In an “s” (slow) process, only a single neutron is absorbed and the product usually decomposes by b-decay into a more proton-rich species.
Elements heavier than iron
26Fe56 + 0n1 \( \rightarrow \) 26Fe56 \( \rightarrow \) 26Fe59 \( \rightarrow \) 27Co59 + –1e0
This process occurs at rates of about 105 yr–1 , and accounts for the lighter isotopes of many elements. The other process (the “r”, or rapid process) occurs in regions of high neutron density and involves multiple captures at rates of 0.1-10 sec–1:
26Fe56 + 13 0n1 \( \rightarrow \) 26Fe56 \( \rightarrow \) 27Co59 + –1e0
This mechanism favors the heavier, neutron-rich isotopes and the heaviest elements.
Other elements
A few nuclei are not accounted for by any of the processes mentioned. These are all low-abundance species, and they probably result from processes having low rates. Examples are Sn112 and Sn114which may be produced through proton-capture, and H2, Li6, Li7, Be, B10 and B11, which may come from spallation processes resulting from collisions of cosmic ray particles with heavier elements
Formation of the solar system
The solar system is believed to have formed about 5 billion years ago as a result of aggregation of cosmic dust and interstellar atoms in a region of space in which the density of such material happened to be greater than average. Over 99.8% of this mass, which consisted mostly of hydrogen, collapsed into a proto-sun; the gravitational energy released in this process raised the temperature sufficiently to initiate the hydrogen fusion reactions discussed above.
The planets
The remaining material probably formed a disk that rotated around the sun. As the temperature dropped to around 2000K, some of the most stable combinations of the elements began to condense out. These substances might have been calcium aluminum silicates, followed by the more volatile iron-nickel system, and then magnesium silicates. The further aggregation of these materials, together with the other constituents of the cooling disk, is now believed to be the origin of the planets. Density estimates indicate that the planets closest to the sun are predominantly rocky in nature, and probably condensed first. The outer planets (Uranus, Neptune and Pluto) appear to consist largely of water ice, methane, and ammonia, with a smaller rocky core.


