Triglycerides
- Page ID
- 444
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\dsum}{\displaystyle\sum\limits} \)
\( \newcommand{\dint}{\displaystyle\int\limits} \)
\( \newcommand{\dlim}{\displaystyle\lim\limits} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\(\newcommand{\longvect}{\overrightarrow}\)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)Triglycerides are esters of fatty acids and a trifunctional alcohol - glycerol (IUPAC name is 1,2,3-propantriol). The properties of fats and oils follow the same general principles as already described for the fatty acids. The important properties to be considered are: melting points and degree of unsaturation from component fatty acids.
Introduction
Since glycerol has three alcohol functional groups, three fatty acids must react to make three ester functional groups. The three fatty acids may or may not be identical. In fact, three different fatty acids may be present. The synthesis of a triglyceride is another application of the ester synthesis reaction. To write the structure of the triglyceride you must know the structure of glycerol and be given or look up the structure of the fatty acid in the table.
| Fat or Oil | Saturated | Unsaturated | |||
|---|---|---|---|---|---|
| Palmitic | Stearic | Oleic | Linoleic | Other | |
| Animal Origin | |||||
| Butter | 29 | 9 | 27 | 4 | 31 |
| Lard | 30 | 18 | 41 | 6 | 5 |
| Beef | 32 | 25 | 38 | 3 | 2 |
| Vegetable Origin | |||||
| Corn oil | 10 | 4 | 34 | 48 | 4 |
| Soybean | 7 | 3 | 25 | 56 | 9 |
| Peanut | 7 | 5 | 60 | 21 | 7 |
| Olive | 6 | 4 | 83 | 7 | - |
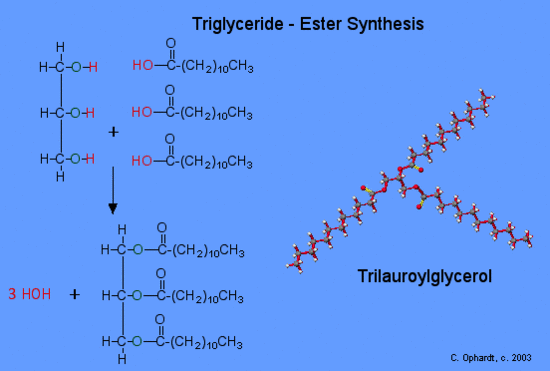
Synthesis of a Triglyceride
Since glycerol, (IUPAC name is 1,2,3-propantriol), has three alcohol functional groups, three fatty acids must react to make three ester functional groups. The three fatty acids may or may not be identical. In fact, three different fatty acids may be present. nThe synthesis of a triglyceride is another application of the ester synthesis reaction. To write the structure of the triglyceride you must know the structure of glycerol and be given or look up the structure of the fatty acid in Table \(\PageIndex{1}\) - find lauric acid.
Glycerol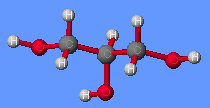
The simplified reaction reveals the process of breaking some bonds and forming the ester and the by product, water. Refer to the graphic on the left for the synthesis of trilauroylglycerol. First, the -OH (red) bond on the acid is broken and the -H (red) bond on the alcohol is also broken. Both join to make HOH, a water molecule. Secondly, the oxygen of the alcohol forms a bond (green) to the acid at the carbon with the double bond oxygen. This forms the ester functional group. This process is carried out three times to make three ester groups and three water molecules.
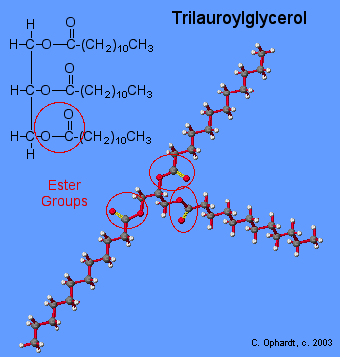
Structure of a Triglyceride
As you can see from the graphic on the left, the actual molecular model of the triglyceride does not look at all like the line drawing. The reason for this difference lies in the concepts of molecular geometry. Trilauroylglycerol. All of the above factors contribute to the apparent "T" shape of the molecule.
Problems
| Quiz: Which acid (short chain or fatty) would most likely be soluble in water? | |
| ... in hexane? |
1 Practice writing out a triglyceride of stearic acid. Again look up the formula of stearic acid and use the structure of glycerol.
2. Write down your answers. Then check the answers from the drop down menu.
| What is the molecular geometry of all three carbons in glycerol (look at model above)? | |
| What is the molecular geometry of the carbon at the center of the ester group? | |
| What is the molecular geometry of the single bond oxygen? |
Outside Links
- Snell, Foster D. "Soap and glycerol." J. Chem. Educ. 1942, 19, 172.
Contributors
- Charles Ophardt, Professor Emeritus, Elmhurst College; Virtual Chembook

