The "Ping-Pong" Mechanism
- Page ID
- 406
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\dsum}{\displaystyle\sum\limits} \)
\( \newcommand{\dint}{\displaystyle\int\limits} \)
\( \newcommand{\dlim}{\displaystyle\lim\limits} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\(\newcommand{\longvect}{\overrightarrow}\)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)The simplest of enzymes will involve one substrate binding to the enzyme and producing a product plus the enzyme. However, the majority of enzymes are more complex and catalyze reactions involving multiple substrates. Binding of two substrates can occur through two mechanisms: sequential mechanism and non-sequential mechanism. In sequential mechanisms both substrates bind the enzyme and the reaction proceeds to form products which are then released from the enzyme. This mechanism can be further subdivided into random and ordered reactions. For random reactions the order in which the substrates bind does not matter. In ordered reactions one substrate must bind the enzyme before the second substrate is able to bind. Non-Sequential mechanism does not require both substrates to bind before releasing the first product. This page will focus on the non-sequential mechanism, which is also known as the "ping-pong" mechanism. It is called this because the enzyme bounces back and forth from an intermediate state to its standard state.The enzyme acts like a ping-pong ball, bouncing from one state to another.
The Mechanism
Ping-pong mechanism, also called a double-displacement reaction, is characterized by the change of the enzyme into an intermediate form when the first substrate to product reaction occurs. It is important to note the term intermediate indicating that this form is only temporary. At the end of the reaction the enzyme MUST be found in its original form. An enzyme is defined by the fact that it is involved in the reaction and is not consumed. Another key characteristic of the ping-pong mechanism is that one product is formed and released before the second substrate binds. The figure below explains the Ping Pong mechanism through an enzymatic reaction.
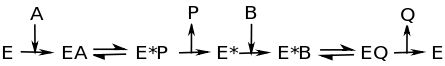
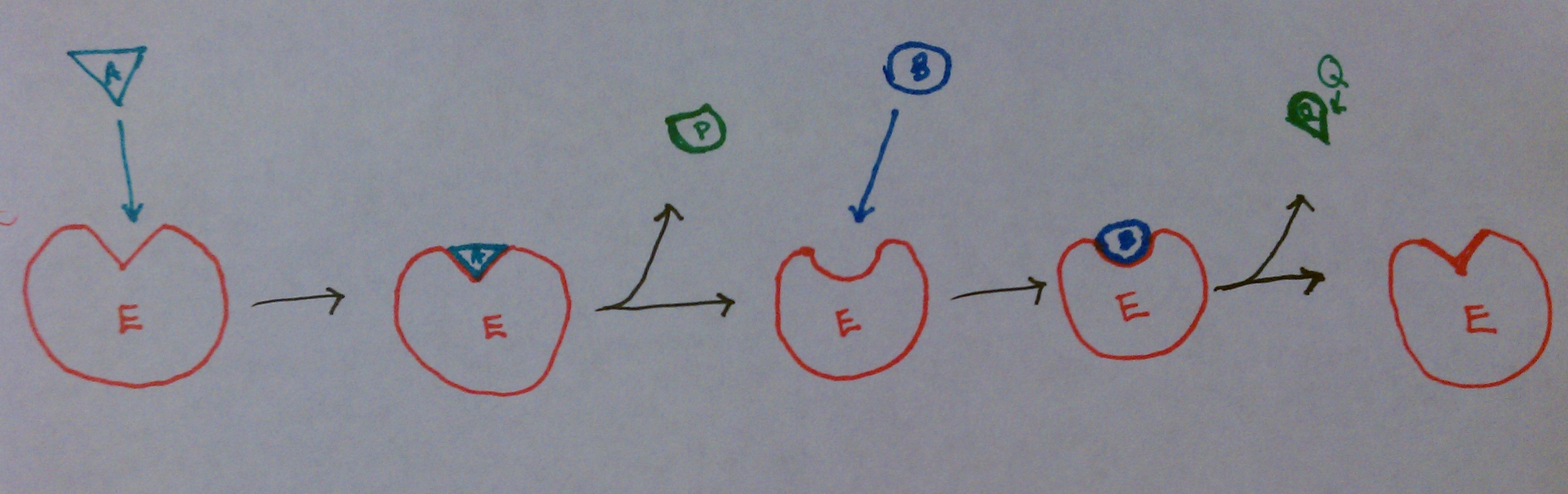
This image shows that as substrate A binds to the enzyme, enzyme-substrate complex EA forms. At this point, the intermediate state, E* forms. P is released from E* , then B binds to E*. B is converted to Q, which is released as the second product. E* becomes E, and the process can be repeated. Often times, E* contains a fragment of the original substrate A.This fragment can alter the function of the enzyme, gets attached to substrate B, or both.
Here is another diagram showing this same reaction:
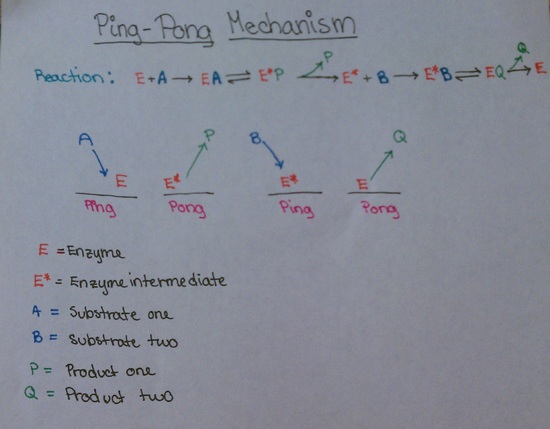
An example of the ping-pong mechanism would be the action of chymotrypsin. When reacted with p-nitrophenyl acetate (A), the reaction of chymotrypsin is seen to occur in two steps. In the first step, the substrate reacts extremely fast with the enzyme, leading to the formation of a small amount of p-nitrophenolate (P). In the second step, the substrate-enzyme interaction results in the formation of acetate ion (Q). The action of chymotrypsin is a ping-pong reaction because the binding of the two substrates causes the enzyme to switch back and forth between two states. Please refer to the section Chymotrypsin and pre-steady-state enzyme kinetics for more details on the action of chymotrypsin.
Another example of an enzyme that exhibits a ping-pong mechanism is pyruvate carboxylase. This enzyme catalyzes the addition of carbon dioxide to pyruvate in order to form oxaloacetate. (leads to gluconeogenesis) This biotin-containing enzyme works by binding CO2 (A) to form carboxybiotin (EA). The biotin swings over towards pyruvate (E*P) and releases CO2. (P, due to the fact that it had been moved from its original binding site) Pyruvate (B), in close proximity to CO2, attacks the partial positive of Carbon in CO2 (E*B). Oxaloacetate is formed within the enzyme (EQ) and gets released (Q). While this attack is occurring, biotin swings back to its initial position, (E* --> E) and is ready to bind another CO2.
Further Reading
An important factor to understand about the ping-pong mechanism is that when plotting a 1/v and 1/[A] plot at varying concentrations of B, a series of parallel lines are seen. In this case A is the first substrate and B being the second substrate.
Refer to these sections on enzyme kinetics and michaelis-menten kinetics to get a better understanding of what this type of plot means.
Sample Questions
- The form in which the ping pong mechanism binds substrates is identified as which type of mechanism?
- What are two characteristics of an enzyme that catalyzes a reaction through the ping-pong mechanism?
- The following diagram shows the mechanism of glutamate-aspartate aminotransferase: Would this mechanism be considered a ping-pong/double-displacement reaction? Why or why not?
Answers
- The ping-pong mechanism is a non-sequential mechanism. A product is released after the first substrate is bound.
- One, a product is seen before the second substrate is bound. Two, binding of the first substrate causes the enzyme to change into an intermediate form that will bind the second substrate. Three, the plot of 1/v vs. 1/[A] as [B] changes will be parallel lines.
- Yes! This would definitely be considered a ping-pong mechanism. First, we can see that there is an E' state which is indicative of a ping-pong mechanism. Pyridoxal is a coenzyme bound to glutamate-aspartate aminotransferase that accepts an amino group from glutamate and becomes pyridoxamine while releasing alpha-ketoglutarate. Pyridoxamine bound to the enzyme will then donate its amino group to oxaloacetate to regenrate pyridoxal as well as aspartate.
References
- Chang, Raymond. 2005. Physical Chemistry for the Biosciences. Sausalito (CA): University Science Books. p. 372-377.
- Cleland, Wallace. "Derivation of Rate Equations for Multisite Ping-Pong Mechanisms with Ping-Pong Reactions at One or More Sites." Journal of Biological Chemistry 248.24 (1973): 8353-355. Print.
-
Garrett, R., and Charles M. Grisham. "Gluconeogenesis, Glycogen Metabolism, and the Pentose Phosphate Pathway." Biochemistry. Belmont, CA: Brooks/Cole, Cengage Learning, 2010. 664-66. Print.
-
Garrett, Reginald and Charles M. Grisham. "Enzymes-Kinetics and Specificity". Biochemistry, Fourth Edition. Belmont, CA: Brooks/Cole, Cengage Learning, 2010. p. 406-407.
Contributors and Attributions
- Melissa Hill, Laura Lan, Andrew Jilwan

