FG10. Quick Reference: Functional Groups and Names
- Page ID
- 137773
The most commonly encountered groups of atoms in organic and biological chemistry are called functional groups. It is useful to be able to recognise these groups, to know what they are called, and to know how to name specific compounds that contain these groups.
For example, compounds that contain the C=C group are called alkenes. Compounds that contain a C=O group, in which the carbon is attached to two other carbons, are called ketones. Alkenes generally have names that end in the suffix "ene", such as pentene, octene, or 2-methyl-2-hexene. Ketones have names that end in "one", such as propanone or butanone.
The table below summarizes a number of these functional groups.
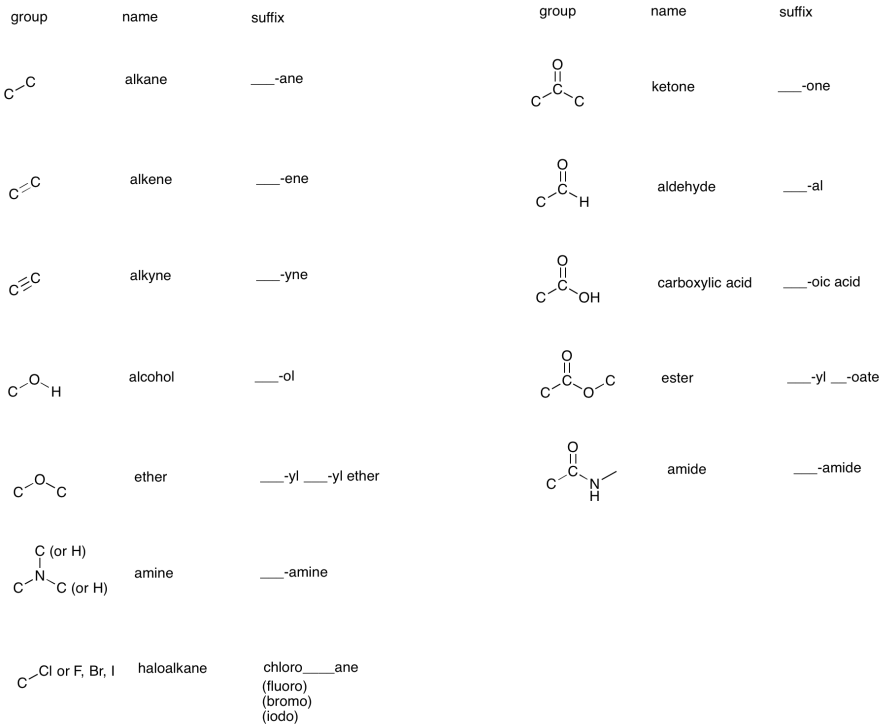
The specific names of organic compounds contain suffixes that tell us about the main functional groups present. The middle part of the name, or base name, tells us about the number of carbons in the main chain of the compound.
The table below outlines how to tell the number of carbons in a main chain or in a group attached to the main chain.
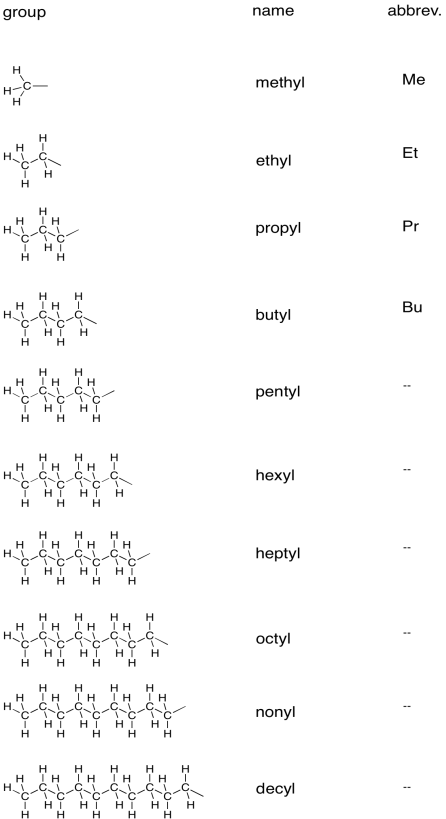
So, a ten-carbon chain containing a C=O group at the end is called decanal. It is an aldehyde, because, being at the end of the chain, the carbonyl carbon is attached to one carbon and one hydrogen. An eight-carbon chain with a C=O group on the third carbon is called 3-octanone. A six-carbon chain attached to an oxygen atom, which is in turn attached to a five-carbon chain, might be called hexyl pentyl ether, provided there are no other complicating features in the structure.
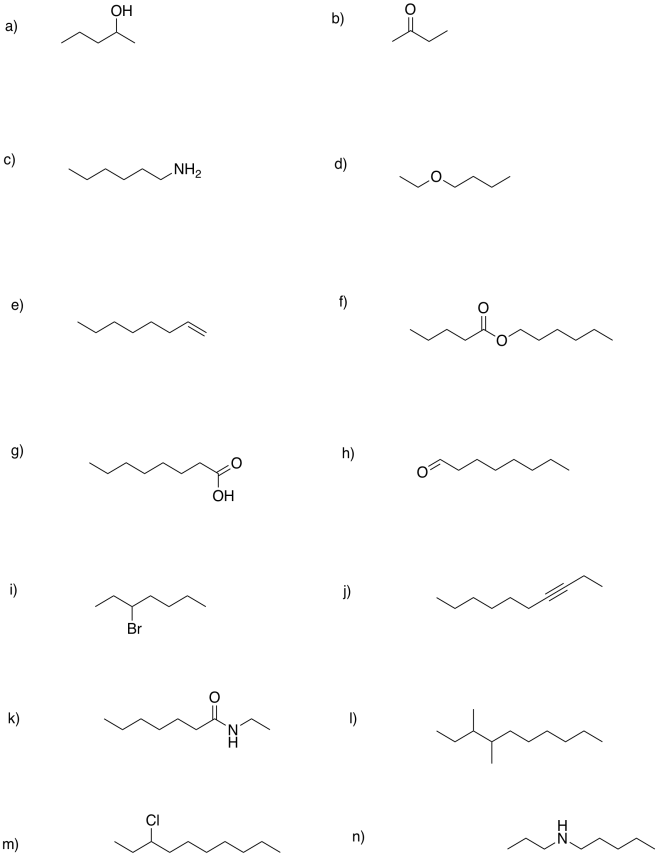
Problem FG10.1.
Use the quick reference to provide names for the following compounds.
Problem FG10.2.
Use the quick reference to provide structures for the following names.
a) ethyl propyl ether b) 4-heptanone
c) 1-chlorohexane d) (E)-3-nonene
e) 2,3,3-trimethylhexane f) hexyl propanoate
g) butanal h) 2-heptanol
i) N-ethyldecylamine j) N-pentylbutanamide
k) pentyl hexanoate l) ethyl pentyl ether
m) 4-bromononane n) 1-octyne
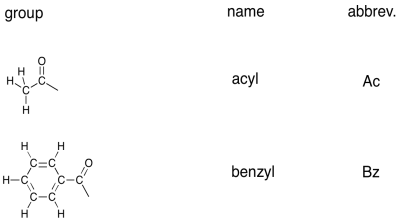
There are a number of variations on these groups that are encountered often enough that they have commonly used names of their own. For example, a 3-carbon group that is attached to another chain via the middle carbon, rather than via the first, is called an isopropyl group.
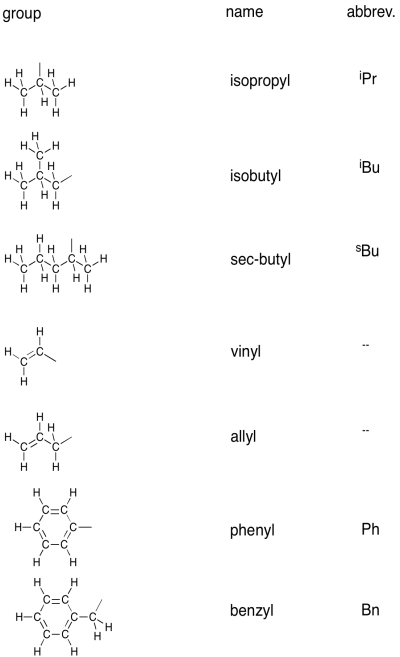
There are a couple of common carbonyl-containing groups, shown below.