FG1. Hydrocarbons
- Page ID
- 137764
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)Hydrocarbons are organic compounds containing only carbon and hydrogen. They include alkanes, alkenes, alkynes and aromatics. The latter three are considered different types of functional groups. Alkanes are not usually considered as functional groups; instead, an alkane is a compound that lacks functional groups.
The functional group in an alkene is a carbon-carbon double bond. The functional group in an alkyne is a carbon-carbon triple bond. Aromatics are cyclic strcutures that are planar, fully conjugated and that possess an odd number of electron pairs in the π bonding system.
It is useful to know the names of the normal (straight-chain) hydrocarbons, in which straight chains of hydrocarbons are connected by single bonds and the carbons' valences are saturated with hydrogens. The names of these compounds form the root names of other compounds having the same number of carbon atoms in a continuous chain. If the end hydrogen in one of these chains is replaces by another atom, the chain is described as a substituent group.
| Number of carbons | Formula | Name | Substituent | Name |
| 1 | CH4 | methane | CH3- | methyl |
| 2 | CH3CH3 | ethane | CH3CH2- | ethyl |
| 3 | CH3CH2CH3 | propane | CH3CH2CH2- | propyl |
| 4 | CH3CH2CH2CH3 | butane | CH3CH2CH2CH2- | butyl |
| 5 | CH3CH2CH2CH2CH3 | pentane | CH3CH2CH2CH2CH2- | pentyl |
| 6 | CH3CH2CH2CH2CH2CH3 | hexane | CH3CH2CH2CH2CH2CH2- | hexyl |
| 7 | CH3CH2CH2CH2CH2CH2CH3 | heptane | CH3CH2CH2CH2CH2CH2CH2- | heptyl |
| 8 | CH3CH2CH2CH2CH2CH2CH2CH3 | octane | CH3CH2CH2CH2CH2CH2CH2CH2- | octyl |
| 9 | CH3CH2CH2CH2CH2CH2CH2CH2CH3 | nonane | CH3CH2CH2CH2CH2CH2CH2CH2CH2- | nonyl |
| 10 | CH3CH2CH2CH2CH2CH2CH2CH2CH2CH3 | decane | CH3CH2CH2CH2CH2CH2CH2CH2CH2CH2- | decyl |
The names of alkenes and alkynes are based on the names of alkanes, but the suffix of the name varies to indicate the functional group present. For example, a two-carbon compound containing a double bond, CH2=CH2, is called ethene rather than ethane.
| Class | Icon | Description | Suffix |
| Alkane | C-C | contains only carbon-carbon single bonds | ane |
| Alkene | C=C | contains a carbon-carbon double bond | ene |
| Alkyne | C=C | contains a carbon-carbon triple bond | yne |
The following scheme provides representative examples of alkanes, alkenes and alkynes as well as a brief guide to how the formal names of these compounds are put together based on their structures. In these simple structures, the length of a carbon chain is indicated in the prefix (see the table above). The presence of double or triple bonds can be indicated by changing the suffix.

Note that alkenes might show cis/trans isomerism. That is, two compounds might have a set of atoms connected in exactly the same order, but with two different arrangements in space. In a trans or E isomer (from the German entgegen, "against"), two groups are found on the opposite sides of the double bond. In the cis or Z isomer (from the German zusammen, "together"), the same two groups are found on the same side of the double bond.
In more complicated structures, the carbon chain branches. In that case, you have to find the main trunk of the structure and name it, then give names to the substituents or branches, telling how long each one of them is and where it is located.
The root name is based on the longest continuous chain. If functional groups are present, the root name is the longest continuous chain that contains the functional group. Substituents are named according to how many carbons they contain in a straight chain, and numbered based on where they are found along the main chain, in which each carbon can be numbered from one end to the other. The lowest possible numbers are used. If a functional group is present, it is always given the lowest possible number.
If several alkyl groups are attached to the main chain, they are listed in alphabetical order in the name of the compound.

Note that, if more than one of the same type of substituent are present, a prfix is used to tell how many of them are present.
| Number | Prefix |
| 2 | di |
| 3 | tri |
| 4 | tetra |
| 5 | penta |
| 6 | hexa |
| 7 | hepta |
Problem FG1.1.
Provide structures for the following alkanes.
a) butane b) heptane c) 2-methyloctane
d) 2,5-dimethylheptane e) 5-ethyl-3-methylheptane f) 2,3,4-trimethylpentane
g) 6-ethyl-2-methyl-4-propyloctane h) 2,2,3,3-tetramethylpentane i) 2,2-dimethyloctane
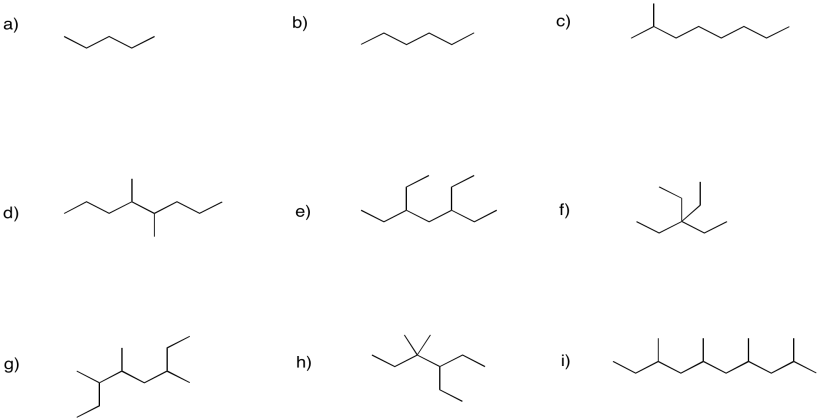
Problem FG1.2.
Provide names for the following alkanes.
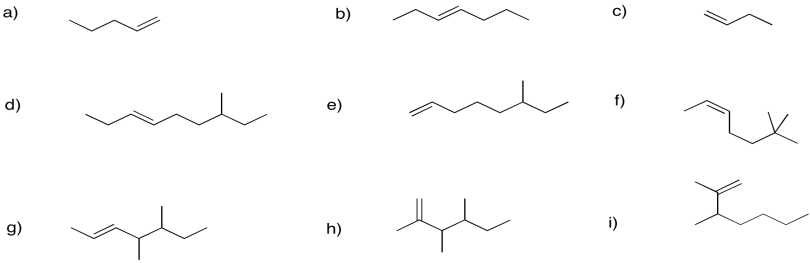
Problem FG1.3.
Provide structures for the following alkenes.
a) 1-decene b) propene c) 2-methylbut-2-ene
d) 2-ethylbut-1-ene e) 3-methylpent-1-ene f) (Z)-4-nonene
g) (E)-3-hexene h) (E)-4-propyldec-2-ene i) (Z)-4-ethyloct-2-ene
Problem FG1.4.
Provide names for the following alkenes.