1: Synthesis of Aspirin (Experiment)
- Page ID
- 61311
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)Over history, many compounds obtained from nature have been used to cure ills or to produce an effect in humans. These natural products have been obtained from plants, minerals, and animals. In addition, various transformations of these and other compounds have led to even more medically useful compounds. During this semester, you will have an opportunity to isolate some pharmacologically active natural products and to synthesize other active compounds from suitable starting materials.
Analgesics are compounds used to reduce pain, antipyretics are compounds used to reduce fever. One popular drug that does both is aspirin. The Merck Index, which is an encyclopedia of chemicals, drugs and biologicals, lists the following information under aspirin: acetylsalicylic acid; monoclinic tablets or needle-like crystals; mp 135 °C (rapid heating); is odorless, but in moist air it is gradually hydrolyzed into salicylic and acetic acids; one gram dissolves in 300 mL of water at 25 °C, in 100 mL of water at 37 °C, in 5 mL alcohol, in 17 mL chloroform.
SYNTHESIS OF ASPIRIN (acetylsalicylic acid)
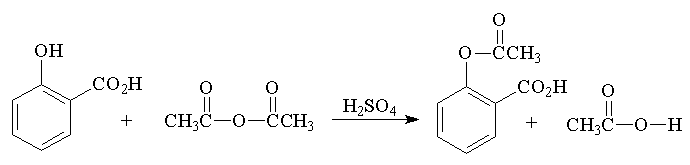
- Place 2.0 g (0.015 mole) of salicylic acid in a 125-mL Erlenmeyer flask.
- Add 5 mL (0.05 mole) of acetic anhydride, followed by 5 drops of conc. H2SO4 (use a dropper, H2SO4 is highly corrosive) and swirl the flask gently until the salicylic acid dissolves.
- Heat the flask gently on the steam bath for at least 10 minutes.
- Allow the flask to cool to room temperature. If acetylsalicylic acid does not begin to crystallize out, scratch the walls of the flask with a glass rod. Cool the mixture slightly in an ice bath until crystallization is completed. The product will appear as a solid mass when crystallization is completed.
- Add 50 mL of water and cool the mixture in an ice bath. Do not add the water until crystal formation is complete.
- Vacuum filter the product using a Buchner funnel. You can use some of the filtrate to rinse the Erlenmeyer flask if necessary.
- Rinse the crystals several times with small portions (5 mL) of cold water and air dry the crystals on a Buchner funnel by suction until the crystals appear to be free of solvent. Test this crude product for the presence of unreacted salicylic acid using the ferric chloride test. Record the weight of the crude solid which probably contains water.
- Stir the crude solid with 25 mL of a saturated aqueous sodium bicarbonate solution in a 150 mL beaker until all signs of reaction have ceased (evolution of \(CO_2\) ceases).
- Filter the solution through a Buchner funnel to remove any insoluble impurities or polymers that may have been formed. Wash the beaker and the funnel with 5 to 10 mL of water.
- Carefully pour the filtrate with stirring, a small amount at a time, into an ice cold HCl solution (ca 3.5 mL of conc. HCl in 10 mL of water) in a 150-mL beaker and cool the mixture in an ice bath. Make sure that the resulting solution is acidic (blue litmus paper) and that the aspirin has completely precipitated out.
- Filter the solid by suction and wash the crystals 3X with 5 mL of cold water each. Remove all the liquid from the crystals by pressing with a clean stopper or cork. Air dry the crystals and transfer them to a watch glass to dry. Test a small amount of the product for the presence of unreacted salicylic acid using the ferric chloride solution.
- When the product is completely dry, weigh the product, determine its melting point (lit mp 135-136 °C) and calculate the percentage yield.
- Dissolve the final product in a minimum amount (no more than 2-3 mL) of hot ethyl acetate in a 25 mL Erlenmeyer flask. Make sure that the product is completely dissolved while gently and continuously heating on a steam bath.
- Cool the solution to room temperature and then in a ice-bath. Collect the product by vacuum filtration and rinse out of the flask with a few milliliters of cold petroleum ether.
- When the product is completely dry, weigh its weight, determine its melting point (lit mp 135 °C) and calculate the percentage yield of this recrystallized product. Calculate the % recovery of recrystallized material from crude material. Submit the crystalline sample in a small vial with proper labeling to your instructor
FERRIC CHLORIDE TEST FOR SALICYLIC ACID
Add 10 drops of aqueous 1% ferric chloride solution to a test tube containing a few crystals of the compound to be tested dissolved in 5 mL water and note the color. Do this test with 1.phenol, 2. salicylic acid, and 3. your crude product. Formation of an iron-phenol complex with Fe(lll) gives a definite color ranging from red to violet, depending upon the particular phenol present.
Questions
- Normally, you measure reactants to at least two significant figures. Why is it not necessary to measure the volume of acetic anhydride to two significant figures? What is the theoretical yield of aspirin (in two significant figures).
- Why is the aspirin washed with cold water?
- According to the data in the Merck Index, if 1.0 g of aspirin is dissolved in 100 mL of water at 37 °C , how much aspirin will precipitate out of solution when it is cooled to 25 °C ?
- A polymeric material, which is a polyester, is formed in this reaction. Polyesters are often made from dicarboxylic acids and diols. In this case, one molecule (salicylic acid) provides both the "alcohol" and the carboxylic acid. Write a structure for the ester formed from acetic acid and ethanol. If you have difficulty, look at the banana oil experiment.
Contributors and Attributions
James Chickos, David Garin, and Valerian D'Souza. University of Missouri–St. Louis; Chemistry)

