2: Synthesis of Acetaminophen (Experiment)
- Page ID
- 61312
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\dsum}{\displaystyle\sum\limits} \)
\( \newcommand{\dint}{\displaystyle\int\limits} \)
\( \newcommand{\dlim}{\displaystyle\lim\limits} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)Acetaminophen - Another pharmacologically active compound
Analgesics are compounds used to reduce pain, antipyretics are compounds used to reduce fever. One popular drug that does both is aspirin, another is acetaminophen which is often used by people who have unwanted, harmful side effects to aspirin. Acetaminophen, which can be synthesized from p-aminophenol, is probably best recognized under the trade name Tylenol. The Merck Index, which is an encyclopedia of chemicals, drugs, and biologicals, lists the following information under acetaminophen: large monoclinic prisms from water, mp 169-170.5, very slightly sol in cold water, considerably more soluble in hot water. Sol in methanol, ethanol, dimethylformamide, acetone, ethyl acetate. Practically insol in petr ether, pentane.
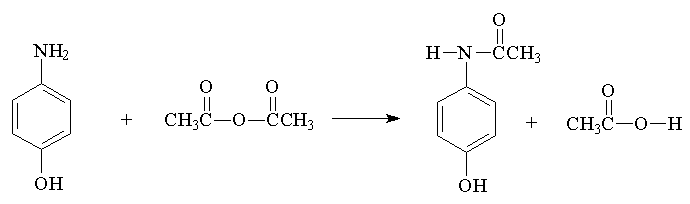
Acetaminophen (CAUTION: p-aminophenol is hazardous to skin, concentrated HCl is caustic)
Practical grade p-aminophenol contains impurities that must be removed at the begining of the synthesis. We will use decolorizing charcoal (Norite) and water for that purpose. The sequence involves first solubilizing the water insoluble amine by converting it into a water soluble amine hydrochloride, then decolorizing. In order to acylate the amine, it is necessary first to neutralize the amine hydrochloride which is accomplished with a sodium acetate buffer, immediately followed by addition of the acylating agent. The neutralization converts the amine hydrochloride back to the free amine which can react with acetic anhydride.
Procedure
Weigh 2.1 g of p-aminophenol into a 125-mL Erlenmeyer flask and add 35 mL of water followed by 1.5 mL of concentrated hydrochloric acid. Swirl the flask in an attempt to dissolve the amine hydrochloride. Add a few more drops of concentrated acid if necessary to dissolve the amine completely as the hydrochloride (it will be difficult to determine since the solution is very dark). Add 0.3-0.4 g of decolorizing charcoal (Norit) to the solution (this is much more than usual but necessary because the crude p-amino-phenol contains a lot of polymeric material), swirl the solution on a steam bath for 4-8 minutes and periodically check to see if the solution is decolorizing (it will be difficult to determine since the solution is dark). Remove the charcoal by gravity filtration into another secured 125-mL Erlenmeyer flask using fluted filter paper while the solution is warm. The flask is secured to prevent tipping. Rinse the filter paper with 1 mL of water. If the charcoal comes through the filter paper it may be necessary to refilter or to use a filter aid, Celite. The filtrate may be clear or, more likely, a tea color. If the solution is a dark brown, add 0.1 g of Norit, heat on the steam bath for a few minutes and filter. The filtrate will darken with time!
While decolorizing the p-aminophenol, prepare a buffer solution by dissolving 2.5 g of sodium acetate trihydrate in 7.5 mL of water which will give 8.8 mL of solution. Clarify the solution by gravity filtration, if necessary.
Warm the filtered aqueous p-aminophenol hydrochloride solution on a steam bath, then add the buffer solution in one portion with swirling. Immediately add 2.0 mL of acetic anhydride while continuing to swirl the solution. Continue heating on the steam bath while swirling vigorously for 10 minutes.
Cool the solution in an ice-water bath, stirring with a glass rod until the crude acetaminophen begins to crystallize. A little bit of rubbing/scratching with a glass rod near the surface often stimulates the crystallization. After crystrallization begins, allow the solution to sit in the ice bath for almost an hour. Filter your product using a Buchner funnel and the water aspirator or house vacuum line. Wash (rinse) the crystals once with a minimum amount of cold water (a few mL should suffice). Allow the crystals to air dry under vacuum. Collect the crude crystalline product and weigh to the nearest tenth of a gram. Record the weight.
Recrystallize all but 100 mg of your crude acetaminophen from water by first dissolving the solid in the minimum amount of hot (boiling) water. Do this carefully adding small amounts of hot water. You do not want to have excess water. Work on a steam bath to keep the solution hot. Add another 2 mL of hot water. If there are no insoluble particles in the solution, you can allow it to cool slowly without having to first filter. If not, decant the hot solution or try to remove the particles with a spatula or Pasteur pipette while keeping the solution warm. After crystallization begins, cool the solution more rapidly using an ice bath. When crystallization ceases (15 minutes), collect the crystals as before, rinsing once with a few mL of cold water, and air drying. Record the weight of the dry, recrystallized acetaminophen and the % recovery from recrystallization (eg; if you obtain 0.75 g recrystallized product after starting with 1.0 g crude product you have 0.75/1.0 x 100 = 75% recovery. Record the percent theoretical yield of dry recrystallized product.
Take a mp of your recrystallized acetaminophen (lit mp 169-170.5).
Contributors and Attributions
James Chickos, David Garin, and Valerian D'Souza. University of Missouri–St. Louis; Chemistry)

