Ionic Mobility and Electrophoresis
- Page ID
- 1391
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)When an electric field is applied to ions in a medium, a phenomenon causes the ions to move with the electric field. The motion of the ion itself can be characterized by charge, shape, and size. The ion moves through the medium and migrates with a characteristic velocity, which is in turn dependent on the characteristics of the ion itself and the properties of the medium. This phenomenon is called electrophoresis.
Introduction
Many different biochemical techniques use the principles of electrophoresis to identify compounds of interest in a sample and understand interactions at the molecular and ionic level. The basic concept of the techniques uses an electric field that attracts or repels certain ions, and measurements of the mobility of the ions through the medium (Figure \(\PageIndex{1}\)). The cations move toward the cathode, and the anions move toward the anode. Heavier or bulkier ions move more slowly through the medium. Visual patterns formed on the various media (through the use of dyes and other techniques, such as Coomassie Brilliant Blue G 250 dye) can be analyzed for useful information.
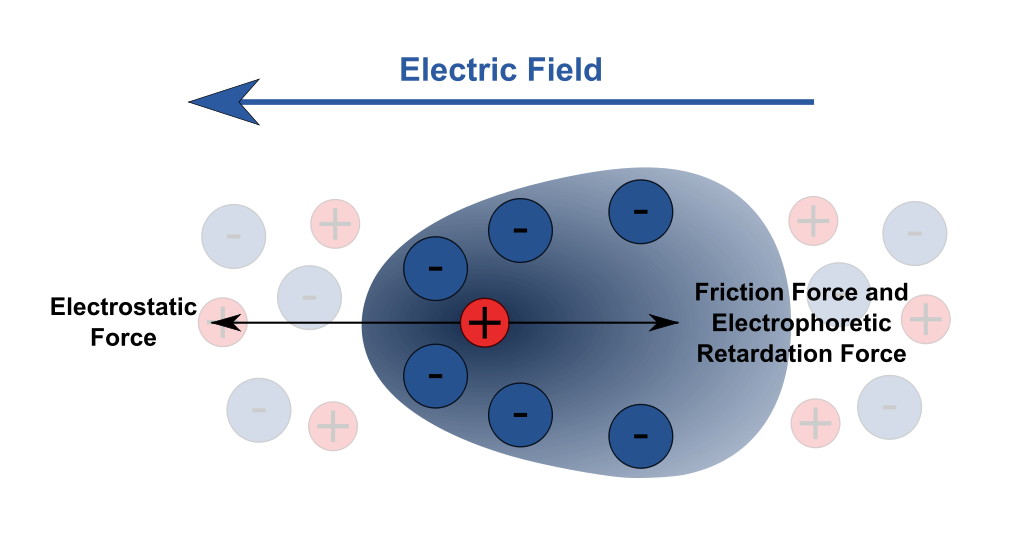
The velocity of the ion is given by the following equation:
\[ v = \dfrac{E \times q}{f} \label{1} \]
where:
- \(v\) is the velocity,
- \(f\) is the coefficient of friction,
- \(E\) is the applied electric field (Volts/cm), and
- \(q\) is the net charge on the ion
The coefficient of friction (\(f\)) in Equation \(\ref{1}\) is introduced due to the fact that when the ion moves through the medium, the medium exerts a frictional force on the ion that inhibits the movement of the ion. Usually the voltage applied is held constant, so the mobility (\(η\)) of the ion can be measured; it is defined as follows:
\[ \eta = \dfrac{v}{E} \label{2} \]
where:
- \(v\) is the velocity
- \(E\) is the applied electric field (Volts/cm)The electric field’s properties determine the separation of ions, and is affected by resistance, current, and voltage.
The higher the current, the faster the migration of the ions (due to increased Coulombic attractions). Because current is affected by voltage, as the difference in potential between the electrodes increases, the rate of migration increases. The resistance is dependent on the properties of the medium; a denser or more saturated medium will retard migration, as will a longer or narrower medium. Mediums with different densities can be made from the same compound, and are quite useful for separating and identifying molecules (through molecular sieving, for example). The most common mediums are agar and polyacrylamide gels.
Of key importance is the buffer used. Different compounds have different stability conditions, and the buffer must be chosen carefully so as not to “harm” the molecules’ native states. The main factors to consider when choosing a buffer are its concentration and its pH. Concentration in terms of electrophoresis buffer refers to the ionic strength of the buffer; a buffer with a higher ionic strength will conduct the current more than the sample, leading to slower migration of the molecules. For compounds that have different ionization forms, pH is a major factor in choosing a buffer. The buffer must have a pH that matches the specific ionization forms’ pH range.
References
- Boyer, Rodney. Modern Experimental Biochemistry (Second Edition). Redwood City: The Benjamin/Cummings Publishing Company, Inc., 1993.
- Williams, Bryan and Wilson, Keith. A Biologist's Guide to Principles and Techniques of Practical Biochemistry. London: Edward Arnold (Publishers) Ltd., 1975
- Plummer, David. An introduction to Practical Biochemistry (Third Edition). London: McGraw-Hill Book Company (UK) Ltd., 1987
Contributors and Attributions
- Sher Butt

