Case Study: Fuel Cells
- Page ID
- 253
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\dsum}{\displaystyle\sum\limits} \)
\( \newcommand{\dint}{\displaystyle\int\limits} \)
\( \newcommand{\dlim}{\displaystyle\lim\limits} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\(\newcommand{\longvect}{\overrightarrow}\)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)Fuels cells generate electricity from an electrochemical reaction in which oxygen and a fuel combine to form water. Fuel cells work by converting the chemical energy found in the fuel, e.g. hydrogen gas, into electrical energy (electricity).
Introduction
In a fuel cell, the fuel is oxidized at the anode which yields electrons that flow through an external circuit doing electrical work, before an oxidant (often oxygen) is reduced by these electrons at the cathode.
\[O_{2(g)} + \underset{\text{Fuel}}{H_{2(g)}} \rightarrow 2H_2O_{(l)} \nonumber \]
The resulting electricity can be used in a variety of ways, including: powering motor vehicles, electrical devices, and airplanes. Fuel cells also produce heat as a by-product which is finding increasing use in heating homes, especially in Japan. With its multifunctional products and by-products, fuel cells are rapidly becoming the hot ticket alternative source of energy as we move into a greener and more progressive era.
What is happening inside a hydrogen fuel cell?
A hydrogen fuel cell enables hydrogen to be combined electrochemically with oxygen to produce electricity, water, and heat. One fuel cell alone only produces a small amount of power. However, grouping the individual fuel cells together creates a fuel cell stack (as shown below in the diagram). When delivered to a fuel cell engine, fuel cell stacks create enough energy to power buses and other vehicles, and have even been used to power spacecraft.
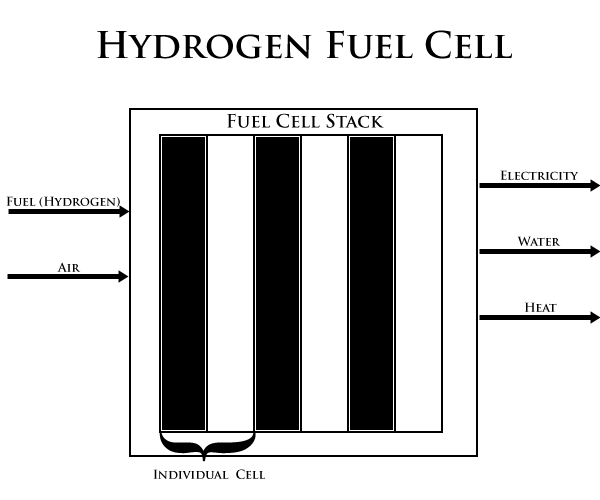
Each fuel cell in the stack contains two electrodes - a positive cathode (where the reduction occurs) and a negative anode (where the oxidation occurs). The energy-producing reactions take place at the surfaces of the electrodes. Each individual pair of electrodes is separated by an electrolyte (either in solid or liquid form). This electrolyte carries electrically charged particles (ions) between the electrodes. The rate of a given reaction can be increased with the help of a catalyst such as platinum or nickel.
The power (\(P\)) produced by a fuel cell is equal to the voltage (\(V\)) multiplied by the current (\(I\)) at which it is being operated. This is measured in watts (W).
\[P= IV \nonumber \]
Factors That Reduce Efficiency in Fuel Cells
There are a number of factors that reduce the efficiency in fuel cells, which can be broadly categorized as reversible, irreversible and fuel utilization losses.
'Reversible' losses correspond to losses that underpin the deviation between the standard electrode potential of the full electrochemical cell and the actual operating open-circuit voltage (OCV).
'Irreversible' losses describe the various contributions from different components of the fuel cell to the loss of voltage with respect to OCV, as the current drawn from the cell/stack is increased. This includes an activation barrier that must be overcome at low currents, which can represent a loss of ~200 mV. This results from the reactions requiring energy to overcome a threshold before occurring, despite a thermodynamic driving force. This is especially relevant in the case of the reduction of oxygen at the cathode. Further losses are experienced in the intermediate regime as a result of ohmic resistance (both ionic and electronic) and due to mass transport limitations at high current loads.
Particularly in the case of PEMFCs, the flows of waste water and unspent fuel occur at rates that exceed the capabilities of the fuel cell's physical capabilities and not all fuel that enters at the inlet is utilized. This also results in a drop of overall efficiency.
Since the efficiency of a fuel cell is directly proportional to the power generated, its efficiency is almost proportional to its voltage.
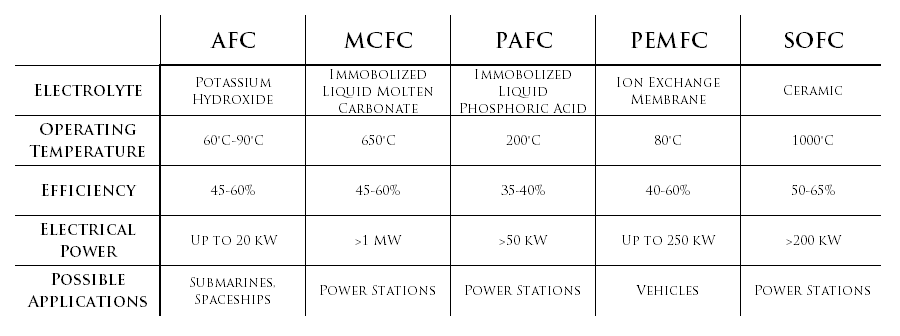
Different Types of Fuel Cells
- Alkaline Fuel Cells (AFC): AFC's use alkaline electrolytes like potassium hydroxide. Generally, a solution of potassium hydroxide in water is used as the electrolyte. The cell operates at 150-200 degrees Celsius, and can generate anywhere from 300 W to 5 MW. AFC's were first used in the Apollo Space Missions by NASA.
- Molten Carbonate Fuel Cells (MCFC): MCFC's use molten carbonate salts as their electrolyte. This fuel cell has a high electrical efficiency of 60 %. These cells operate at about 600 degrees Celsius. The generated power varies, and some units have been built with outputs as high as 100 MW. Because of the high temperatures, these cells are not generally used in the home.
- Zinc Air Fuel Cells (ZAFC): In this type of fuel cell, there is a gas diffusion electrode, a zinc anode separated by electrolyte and a mechanical separator. Oxygen is reduced to hydroxide, which combines with oxidized zinc, generating electrons in the process.
- Phosphoric Acid Fuel Cells (PAFC): This fuel cell's anode and cathode are made with specks of platinum on a carbon and silicon carbide matrix that supports the phosphoric acid electrolyte. PAFC's are commonly used in large commercial vehicles i.e. buses and were the first fuel cells to be commercialized.
- Proton Exchange Membrane Fuel Cells (PEMFC): This fuel cell uses a polymeric membrane as the electrolyte along with platinum-activated, carbon-based electrodes. PEMFC's can function at relatively low temperatures and are therefore commonly used in scenarios requiring short start-up times, including transport and portable applications.
- Direct Methanol Fuel Cells (DMFC): This fuel cell is similar to the Proton Exchange Membrane Fuel Cell; however, instead of using gaseous hydrogen as the fuel, liquid methanol is used.
- Solid Oxide Fuel Cells (SOFC): SOFC's can operate from ~ 550 °C to 1000 °C. SOFC's are able to do so because they use a solid ceramic electrolyte between their electrodes. Like MCFC's, SOFC's can also perform at an efficiency of around 60 %. These fuel cells are often used for generating heat and electricity in industry and for providing auxiliary power in motor vehicles.
The Benefits of Fuel Cell Power
Fuel cells create little to no environmentally damaging emissions: generally, the only byproduct in a hydrogen fuel cell, typically found in automobiles, is water.
- As long as fuel is always available, the fuel cell can run for an infinite amount of time.
- Fuel cells offer cleaner, quieter, and more efficient power production than conventional internal combustion engines.
- Fuel cells can use a variety of fuels, such as: natural gas, methanol, gasoline, and hydrogen. Fuel cells are becoming increasingly reliable, with some systems demonstrating very low degradation rates.
Where are Fuel Cells Being Used Now?
Fuel cells are being used all over the globe. Here are some examples:
- NASA was the first to use fuel cells commercially in the 60's. In fact alkaline fuel cells have flown over 100 missions and been used for over 80,000 hours for NASA.
- The US Navy has used fuel cells in submarines since the 80's.
- Backed by the European Union, Clean Urban Transportation for Europe, "CUTE", fuel cell buses have been successfully implemented in various European cities.
- As part of its national project to create a fossil fuel free economy, Iceland, has begun to convert its fishing fleet from diesel engines to hydrogen fuel cells.
- Fuel cells are also being designed to be able to power smaller electronics such as cellular phones, laptop computers, and also for use as an auxiliary power source. Portable mobile phone chargers are now commercially available.
- Many major car manufacturers all over the world have already created various prototypes of fuel cell powered automobiles.
Outside links
- Online Encyclopedia Article: en.Wikipedia.org/wiki/Fuel_cell
- Video Of How It Works: www.youtube.com/watch?v=esuAlB4NVi0
- The Chemistry: www.princeton.edu/~chm333/200...hemistry.shtml
- The History: www.princeton.edu/~chm333/200...-history.shtml
References
- Petrucci, Ralph H. General Chemistry: Principles and Modern Applications 9th Ed. New Jersey: Pearson Education Inc. 2007.
- Koppel, Tom. Powering the Future : The Ballard Fuel Cell and the Race to Change the World. New York: John Wiley & Sons, Incorporated, 1999.
Problems
- Which of the following fuel cells is the most efficient?
- Solid Oxide Fuel Cell (SOFC)
- Proton Exchange Membrane Fuel Cell (PEMFC)
- Molten Carbonate Fuel Cell (MCFC)
- Alkaline Fuel Cell (AFC)
- Phosphoric Acid Fuel Cell (PAFC)
- Name one byproduct of an active hydrogen fuel cell.
- What company/organization was the first to commercialize fuel cells?
- What role does an electrolyte play in a fuel cell?
- Name one way in which fuel cells are beneficial to society.
Solutions
1. Answer: A) Solid Oxide Fuel Cell (SOFC)
Explanation: As explained by the table in the 'Different Types of Fuel Cells' section, the Solid Oxide Fuel Cell (SOFC) is the most efficient with an efficiency of 50-65%.
2. Answer: Water OR Heat
Explanation: Both water and heat are by-products of an active hydrogen fuel cell.
3. Answer: United Technologies Corporation
Explanation: United Technologies Corporation was the first company to commercialize fuel cells.
4. Answer: It serves as a bridge between anode and cathode.
Explanation: An electrolyte serves a bridge that connects the anode and cathode parts of a fuel cell. This is because of the ionic nature of salts in solution. As a voltage is applied, these ions align either at the cathode or at the anode according to their charge. This creates a bridge that the voltage can cross.
5. Answer: No (very little) carbon-dioxide emissions OR Very high efficiency
Explanation: The very efficient nature of fuel cells relative to standard combustion engines and other similar devices is highly valued in today's society because of the rising cost of energy. The relatively low emissions produced by fuel cells is also beneficial to the environment.

