Introduction to Sample Preparation
- Page ID
- 154861
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)Overview
This module introduces some of the basics of sample preparation, and the ways in which samples must be handled and modified to make them amenable to a particular instrumental method of analysis. Included are theory and applications of liquid-liquid and solid phase extraction and microextraction techniques, and derivatization methods for GC and HPLC. Throughout the module, the primary goal is to present thought-provoking questions that lead to critical thinking about which of the available sample preparation methods is appropriate for a given sample scenario. Supporting materials detailing these techniques can be found in Chapter 7 in the online textbook Analytical Chemistry 2.01 by David Harvey available from the Analytical Sciences Digital Library (www.asdlib.org ). The final section of Chapter 7 contains key terms and links to further discussion. If you need to review any terminology, the final section of Chapter 3 in Harvey also defines many of the key terms used in this module.2
After completion of this learning module, you will be able to:
- Describe why many samples need some form of preparation before analysis.
- Describe the properties of an analyte that can be used to separate it from a mixture.
- Describe the general theory of the following extraction techniques
- Liquid-liquid extraction (LLE)
- Solid-phase extraction (SPE)
- Adsorption
- Charge (anion/cation exchange)
- Mixed modes
- QuEChERS
- Solid-phase microextraction
- Describe common derivatization methods used in sample preparation methods for gas chromatography
- Describe common derivatization methods that “tag” an analyte, allowing it to be detected spectrophotometrically, electrochemically or using fluorescence
- Predict the best extraction method for isolating the analyte given a sample scenario.
Introduction
Consider your sample as shown in Figure 1, comprised of your analyte (triangles) in a matrix of various interferents (circles and moons). Consider someone trying to measure neonicotinoid pesticides in a plant sample collected in an area with a noticeable bee decline. These pesticides have in recent years come under scrutiny for contributing to the decline in honeybee populations.3 A field is sprayed with a neonicotinoid pesticide. You take some plant samples back to the lab and homogenize for subsequent preparation and analysis.
Q1. Do an Internet search to find structures of nicotine and imidacloprid (one of the neonicotinoids). Do these structures indicate to you how this class of insecticides was named?
Q2. In the plant sample, state the identity of your analyte and list some possible interferents.
The interferents can cause problems with your eventual measurement by doing things such as
- Adding to the analyte signal
- Masking the analyte signal
- Quenching the analyte’s signal
- Wreaking havoc on your instrument (e.g. clogging a chromatography column)
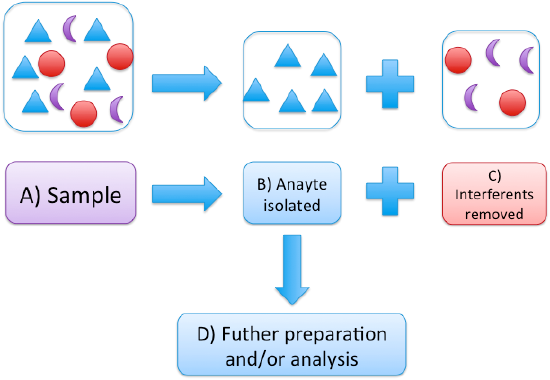
Figure 1: Shows the removal of interferents (circles and moons) from the analyte (triangles) in a complex sample (A). After isolation (B), the sample may be ready for analysis, or additional preparation steps (like concentration or derivatization) may be required.
Q3. List at least four chemical properties of the analyte that may allow it to be separated from interfering components in its matrix.
Keep your list in mind as you read through the next sections describing various extraction methodologies.
Sample preparation
|
A) How do you get your sample from this… |
B) To this? |
|
|
|
Figure 2. Photographs of samples before (A) and after (B) the preparation steps for injection onto a gas chromatograph.
As shown in Figure 2, in most chemical analyses, the sample as collected is not suitable for direct analysis, but must be converted into a proper form through a process known as sample preparation. For example, a solid may have to be dissolved in water or digested in acid, and then filtered to remove insoluble components. Compounds that could interfere with the analysis must be removed or masked during sample preparation; a process that typically involves a separation step. Also, within this step it may be necessary to dilute or concentrate the sample to place the analyte within the appropriate concentration dynamic range of the measurement.
In this module several aspects of sample preparation will be considered. The focus of the first section is directed at liquid-liquid extraction (LLE) and solid phase extraction (SPE). Though other aspects of sample preparation such as digestion, precipitation, and pre-concentration are critical to a successful analysis, they are typically specific for the sample and analyte to be determined. On the other hand, methods of extraction can be considered in more general terms, and their successful exploitation often determines the success of a validated analytical method. Figure 3 below outlines the extraction methods discussed in this series of modules.
Figure 3. Diagram of the extraction methods discussed in this module series.
Additionally, even after purification, samples may not be in a form that is amenable to determination by typical instrumental methods. In many cases, the sample must be converted by reaction to a form containing an additional functionality detectable by the instrument of choice. This conversion, referred to as derivatization will be discussed in sections that address sample analysis by GC and by HPLC.
References, Introduction
1. See Chapter 7 “Collecting and Preparing Samples” in Analytical Chemistry 2.0
http://www.asdlib.org/onlineArticles/ecourseware/Analytical%20Chemistry%202.0/Text_Files_files/Chapter7.pdf#page=31&zoom=auto,-32,324 (accessed September 13, 2018)
2. Chapter 3 “The Vocabulary of Analytical Chemistry” in Analytical Chemistry 2.0
http://www.asdlib.org/onlineArticles/ecourseware/Analytical Chemistry 2.0/Text_Files_files/Chapter3.pdf (accessed September 13, 2018)
3. Erickson, B.E. “White House Moves to Save Bees”, Chemical and Engineering News, June 23, 2014
cen.acs.org/articles/92/web/2014/06/White-House-Moves-Save-Bees.html (accessed September 13, 2018)






