1.1: Electronic transitions and luminescence
- Page ID
- 76095
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)Luminescence is the emission of light due to transitions of electrons from molecular orbitals of higher energy to those of lower energy, usually the ground state or the lowest unoccupied molecular orbitals. Such transitions are referred to as relaxations. Figure A1.1 shows four electronic energy levels (S0,S1, S2 and T1) and the possible transitions between them. S0 represents the ground state, while S1, S2 and T1 represent higher-energy excited states;S0, S1 and S2 are singlet states in which all the electrons form pairs of opposed spins whereas T1 is a triplet excited state, in which not all electrons are paired off in this way.
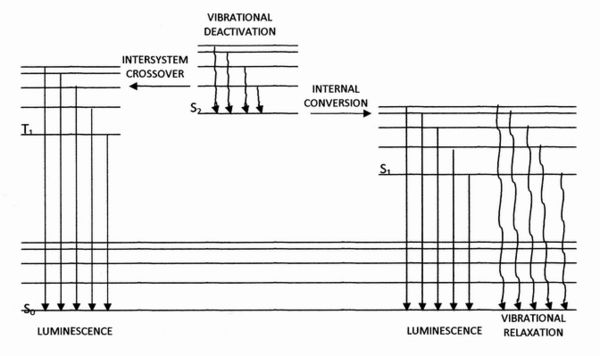
Figure A1.1 – Jablonski diagram showing four electronic energy levels S0, S1, S2 and T1, with their vibrational fine structure and the transitions between them that affect luminescence.
Each energy level is subdivided into a number of vibrational states, each characterised by an amount of vibrational energy that accompanies the potential energy of the electrons occupying the orbitals. Luminescence is classified according to the excited state that gives rise to it and to the source of the energy that caused the excited state to be populated with electrons. The promotion of electrons to an excited state is called excitation. In many cases, this is brought about by absorption of visible or ultraviolet radiation. In such a case, if the luminescence arises because electrons are relaxing from a singlet excited state to a singlet ground state, then it is called fluorescence, and generally occurs within 10-11 to 10-5 s. The transition is very fast because it involves no reversal of electron spin. If, however, it arises due to relaxation from a triplet excited state, then the luminescence is called phosphorescence, which generally occurs within 10-4 to 100 s. If the excitation is the result of energy released in a chemical reaction, the luminescence is called chemiluminescence. A subset of chemiluminescence occurring in the biosphere as a result of biological processes is called bioluminescence. Electrochemiluminescence is another distinct subset of chemiluminescence phenomena, made up of those reactions in which the excited species is produced at an electrode during electrolysis.
Before luminescence occurs, there is a non-radiative loss of energy (due to collisions between molecules) as the excited state relaxes to a lower vibrational state while remaining at the same electronic energy level. This type of transition is called vibrational deactivation. It has to occur even more rapidly than fluorescence and typically occurs within 10-12 s of excitation. Therefore the luminescence involves the emission of photons of lower energy (higher wavelength) than would otherwise be the case. Another possible transition is internal conversion, in which an electron transfers from a lower vibrational state of a higher electronic energy level to a higher vibrational state of a lower electronic energy level, without any significant gain or loss of energy; such a transition, S2 → S1, is shown schematically in Figure 1.1. In intersystem crossing, internal conversion would involve also reversal of the spin of the electron as in a transition from a singlet to a triplet state; the transition S2 → T1 in Figure 1.1 is of this type. Such transitions can give rise to phosphorescence. Finally, luminescence is not inevitable. The intensity of the emission compared with the number of molecules in the excited state is called the quantum yield(ΦF). This can be calculated for fluorescent emission by dividing the number of emitted photons by the number of absorbed photons. ΦF in a chemiluminescence phenomenon should be the same as in the fluorescence phenomenon involving the same excited state, but, because chemiluminescence does not depend on the absorption of photons, it can be calculated in the same way only by performing a separate fluorescence experiment. The intensity of chemiluminescence emission is more meaningfully compared with the number of reactant molecules; this measure is called the chemiluminescence quantum yield (ΦCL). It is related to ΦF by the equation:
\[Φ_{CL} = Φ_CΦ_EΦ_F \nonumber \]
where ΦC is the proportion of reactant molecules converted into product and ΦE is the proportion of product molecules formed in the excited state. ΦCL has values from 0 to 1 and reaches 0.88 for firefly luciferin in vitro[1].
Because ΦCL depends on ΦF it would be reasonable to suppose that chemiluminescence is affected by substitution in product molecules in the same way as is fluorescence. In that case, ΦCLwould be increased by electron donors and decreased by electron acceptors. There would also be an increase in ΦCL (and a bathochromic shift in emission wavelength) due to conjugated systems and in rigidly planar molecules having facilitated π-bond delocalisation. Such generalisations must be used with great care, for the only product species to which they can apply are the molecules that are actually emitting; it is by no means obvious what these are in any particular case.
This content originates from an Analytical Chemiluminescence Wikibook and is licensed via a Creative Commons Attribution-ShareAlike License.

