1.1: What is Analytical Chemistry
- Page ID
- 127056
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)This quote is attributed to C. N. Reilly (1925-1981) on receipt of the 1965 Fisher Award in Analytical Chemistry. Reilly, who was a professor of chemistry at the University of North Carolina at Chapel Hill, was one of the most influential analytical chemists of the last half of the twentieth century.
For another view of what constitutes analytical chemistry, see the article “Quo Vadis, Analytical Chemistry?”, the full reference for which is Valcárcel, M. Anal. Bioanal. Chem. 2016, 408, 13-21.
Let’s begin with a deceptively simple question: What is analytical chemistry? Like all areas of chemistry, analytical chemistry is so broad in scope and so much in flux that it is difficult to find a simple definition more revealing than that quoted above. In this chapter we will try to expand upon this simple definition by saying a little about what analytical chemistry is, as well as a little about what analytical chemistry is not.
Analytical chemistry often is described as the area of chemistry responsible for characterizing the composition of matter, both qualitatively (Is there lead in this paint chip?) and quantitatively (How much lead is in this paint chip?). As we shall see, this description is misleading.
Most chemists routinely make qualitative and quantitative measurements. For this reason, some scientists suggest that analytical chemistry is not a separate branch of chemistry, but simply the application of chemical knowledge [Ravey, M. Spectroscopy, 1990, 5(7), 11]. In fact, you probably have performed many such quantitative and qualitative analyses in other chemistry courses.
You might, for example, have determined the concentration of acetic acid in vinegar using an acid–base titration, or used a qual scheme to identify which of several metal ions are in an aqueous sample.
Defining analytical chemistry as the application of chemical knowledge ignores the unique perspective that an analytical chemist bring to the study of chemistry. The craft of analytical chemistry is found not in performing a routine analysis on a routine sample—a task we appropriately call chemical analysis—but in improving established analytical methods, in extending these analytical methods to new types of samples, and in developing new analytical methods to measure chemical phenomena [de Haseth, J. Spectroscopy, 1990, 5(7), 11].
Here is one example of the distinction between analytical chemistry and chemical analysis. A mining engineers evaluates an ore by comparing the cost of removing the ore from the earth with the value of its contents, which they estimate by analyzing a sample of the ore. The challenge of developing and validating a quantitative analytical method is the analytical chemist’s responsibility; the routine, daily application of the analytical method is the job of the chemical analyst.
- Conception of analytical method (birth).
- Successful demonstration that the analytical method works.
- Establishment of the analytical method’s capabilities.
- Widespread acceptance of the analytical method.
- Continued development of the analytical method leads to significant improvements.
- New cycle through steps 3–5.
- Analytical method can no longer compete with newer analytical methods (death).
Steps 1–3 and 5 are the province of analytical chemistry; step 4 is the realm of chemical analysis.
The seven stages of an analytical method listed here are modified from Fassel, V. A. Fresenius’ Z. Anal. Chem. 1986, 324, 511–518 and Hieftje, G. M. J. Chem. Educ. 2000, 77, 577–583.
Another difference between analytical chemistry and chemical analysis is that an analytical chemist works to improve and to extend established analytical methods. For example, several factors complicate the quantitative analysis of nickel in ores, including nickel’s unequal distribution within the ore, the ore’s complex matrix of silicates and oxides, and the presence of other metals that may interfere with the analysis. Figure 1.1.1 outlines one standard analytical method in use during the late nineteenth century [Fresenius. C. R. A System of Instruction in Quantitative Chemical Analysis; John Wiley and Sons: New York, 1881]. The need for many reactions, digestions, and filtrations makes this analytical method both time-consuming and difficult to perform accurately.
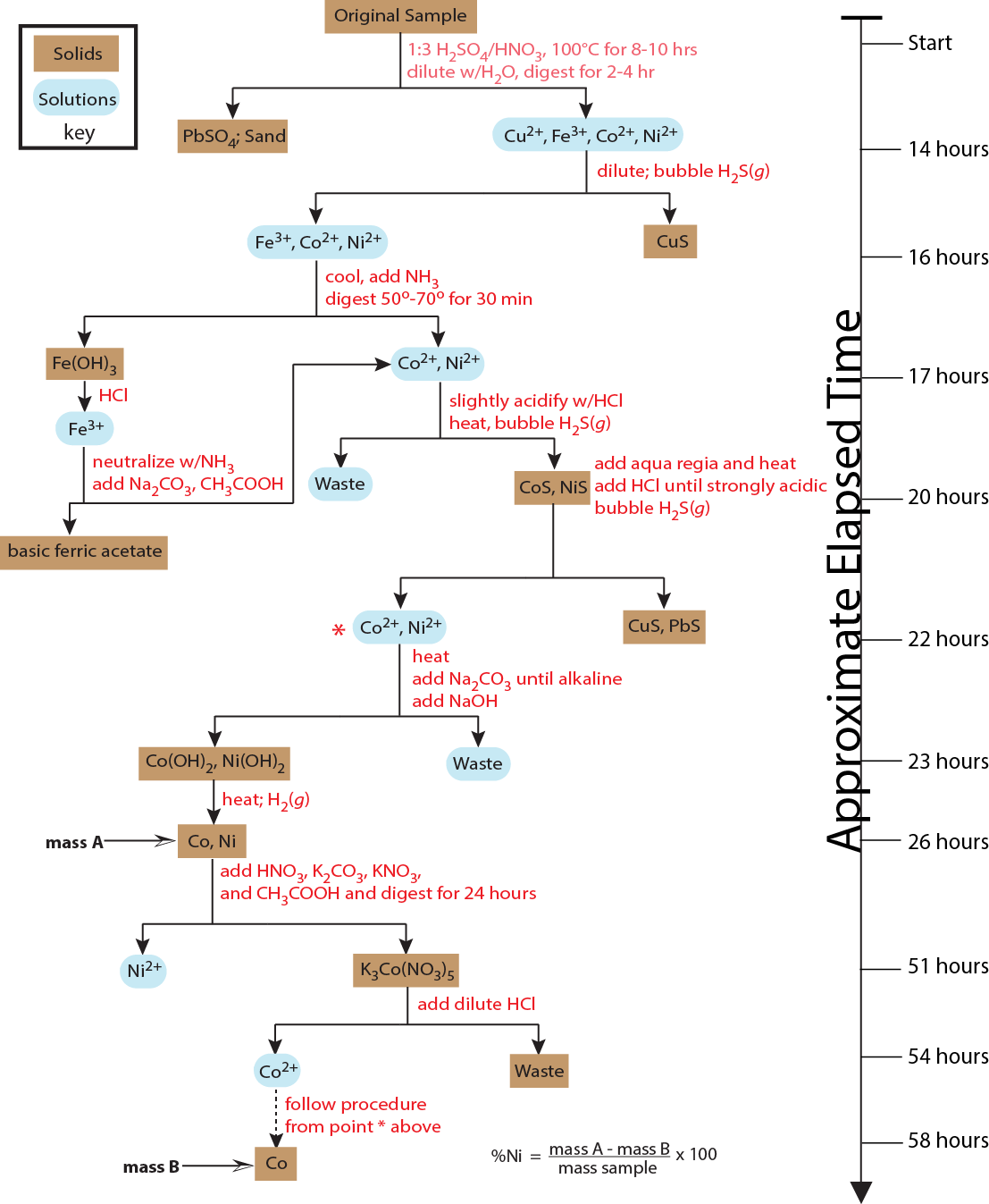
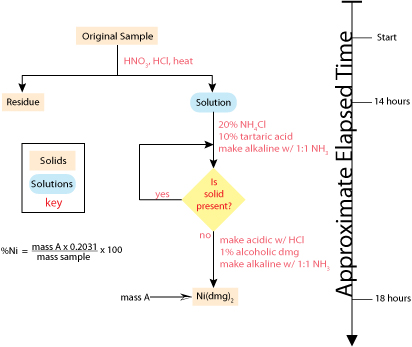
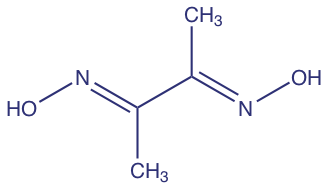
The discovery, in 1905, that dimethylglyoxime (dmg) selectively precipitates Ni2+ and Pd2+ led to an improved analytical method for the quantitative analysis of nickel [Kolthoff, I. M.; Sandell, E. B. Textbook of Quantitative Inorganic Analysis, 3rd Ed., The Macmillan Company: New York, 1952]. The resulting analysis, which is outlined in Figure 1.1.2 , requires fewer manipulations and less time. By the 1970s, flame atomic absorption spectrometry replaced gravimetry as the standard method for analyzing nickel in ores, resulting in an even more rapid analysis [Van Loon, J. C. Analytical Atomic Absorption Spectroscopy, Academic Press: New York, 1980]. Today, the standard analytical method utilizes an inductively coupled plasma optical emission spectrometer.
Perhaps a more appropriate description of analytical chemistry is “the science of inventing and applying the concepts, principles, and...strategies for measuring the characteristics of chemical systems” [Murray, R. W. Anal. Chem. 1991, 63, 271A]. Analytical chemists often work at the extreme edges of analysis, extending and improving the ability of all chemists to make meaningful measurements on smaller samples, on more complex samples, on shorter time scales, and on species present at lower concentrations. Throughout its history, analytical chemistry has provided many of the tools and methods necessary for research in other traditional areas of chemistry, as well as fostering multidisciplinary research in, to name a few, medicinal chemistry, clinical chemistry, toxicology, forensic chemistry, materials science, geochemistry, and environmental chemistry.
To an analytical chemist, the process of making a useful measurement is critical; if the measurement is not of central importance to the work, then it is not analytical chemistry.
You will come across numerous examples of analytical methods in this textbook, most of which are routine examples of chemical analysis. It is important to remember, however, that nonroutine problems prompted analytical chemists to develop these methods.
An editorial in Analytical Chemistry entitled “Some Words about Categories of Manuscripts” highlights nicely what makes a research endeavor relevant to modern analytical chemistry. The full citation is Murray, R. W. Anal. Chem. 2008, 80, 4775; for a more recent editorial, see “The Scope of Analytical Chemistry” by Sweedler, J. V. et. al. Anal. Chem. 2015, 87, 6425.


