6.5: Radical Chain Reactions: Propagation
- Page ID
- 148718
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)Name: ______________________________
Section: _____________________________
Student ID#:__________________________
Template:HideTOCRadicals are known for engaging in "chain reactions". In a chain reaction, a reactive intermediate is generated. When it reacts, it leaves another reactive intermediate, much like the first. This event is called "propagation".
There are a couple of common ways that propagation occurs. The radical might achieve its stable electron count by snatching another atom, especially a hydrogen atom. That event is called hydrogen atom abstraction. Alternatively, a radical may bond with one of the electrons in a pi bond.
In the abstraction of an atom, the radical forms a bond with that atom. That bond gives the radical an even number of electrons again.
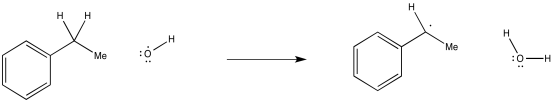
Problem RR5.1.
Show, with arrows, the mechanism for abstraction of a hydrogen atom from ethylbenzene by a hydroxyl radical.
It's important to note that in a hydrogen atom abstraction, the radical is reacting not just with the proton, but with the entire hydrogen atom. It is taking the electron, too.
Exactly which atom gets abstracted has a lot to do with bond strengths. For example, O-H bonds are quite strong (up to 120 kcal/mol, in water, for example). Thus, an OH radical will frequently abstract hydrogen atoms, because there is an energetic payoff when that happens.
Of course, a bond also has to get broken during an abstraction. That costs some energy. C-H bonds are also pretty strong, so they may be hard to break. However, some C-H bonds in particular are weaker than others. For example, in order to break a benzylic C-H bond, the cost is only about 88 kcal/mol. In the case of hydrogen atom abstraction from ethylbenzene by hydroxyl radical, the trade-off is worth it.
Problem RR5.2.
Draw a reaction progress diagram for the abstraction of a hydrogen atom from ethylbenzene by a hydroxyl radical.
It is not always the case that a reaction is purely determined by the thermochemistry of the bonds involved. Sometimes, there are kinetic factors that block the path to the more stable product, or that lower the path to the less stable product. However, in many atom abstractions, because the old bond is being broken at the same time that the new bond is being formed, both factors matter in the rate determining step. By the time the transition state is reached, the stability of the complex is influenced both by the bond that is being broken and the bond that is being made. As a result, the thermodynamics of the reaction can have a strong influence on the pathway to products.
| Bond | Dissociation Energy (kcal/mol) | Bond | Dissociation Energy (kcal/mol) |
| F-H | 136 | Br-H | 88 |
| Cl-H | 103 | I-H | 71 |
| EtO-H | 105 | O2N-OMe | 42 |
| CH3S-H | 87 | Cl-OMe | 48 |
| PhO-H | 87 | H3C-OMe | 85 |
| Me2N-H | 91 | H3C-NH2 | 85 |
| Et3Si-H | 96 | H3C-F | 115 |
| Bu3Ge-H | 88 | CH3-H2C-Cl | 85 |
| Bu3Sn-H | 78 | CH3-H2C-Br | 72 |
| Me3Sn-Cl | 100 | CH3-H2C-I | 57 |
Problem RR5.3.
Indicate whether a dimethylamine radical is likely to carry out hydrogen atom abstraction from each of the following molecules.
a) Et3SiH b) PhOH c) EtOH d) Bu3SnH e) HF f) HI
Problem RR5.4.
Indicate whether a chlorine atom abstraction would be likely to occur in each of the following cases.
- Chloroethane is exposed to methoxy radical.
- Chloroethane is exposed to trimethyltin radical.
- Trimethyltin chloride is exposed to methoxy radical.
Problem RR5.6.
Explain why a trialkyltin radical (R3Sn) would not be able to remove a hydrogen atom from propane, but could abstract a chlorine atom from chloroethane.
Addition to an alkene is another common propagation pathway in radical reactions. In this case, a π (pi) bond is broken in the alkene to form a new bond to the radical species. That leaves the second electron from the π bond to form a new radical species.

Π bonds are often weaker than σ bonds, making this pathway energetically accessible in many cases. Perhaps more importantly, the electrons in π bonds are found above and below a flat part of the molecule, leaving them open and accessible for reaction with radicals.
| Bond | Bond dissociation energy (kcal/mol) | Bond | Bond dissociation energy (kcal/mol) |
| H3C-CH3 | 90 | H2C=CH2 | 174 |
| H3C-NH2 | 85 | H2C=NH | ? |
| H3C-OH | 92 | H2C=O | 179 |
Problem RR5.7.
Show a mechanism, with curved arrows, for the reaction of pentene with bromine atom.
Problem RR5.8.
Calculate the strengths of the following pi bonds.
- In ethene.
- In methanal.
Problem RR5.9.
The pi bond in PhCH=NPh has been calculated to have a dissociation energy of 77 kcal/mol.
- Estimate the missing imine C=N bond dissociation energy in the above table.
- Explain why your estimate may be unreliable.


