4: Activity Series of Metals
- Page ID
- 149245
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)Name: ______________________________
Section: _____________________________
Student ID#:__________________________
Template:HideTOCActivity series data table1
| Metal | Ion Formed | Reactivity |
| Cs | Cs+ |
reacts with water |
| Rb | Rb+ | |
| K | K+ | |
| Na | Na+ | |
| Li | Li+ | |
| Ba | Ba2+ | |
| Sr | Sr2+ | |
| Ca | Ca2+ | |
| Mg | Mg2+ |
reacts with acids like HCl |
| Al | Al3+ | |
| Mn | Mn2+ | |
| Zn | Zn2+ | |
| Cr | Cr2+ | |
| Fe | Fe2+ | |
| Cd | Cd2+ | |
| Co | Co2+ | |
| Ni | Ni2+ | |
| Sn | Sn2+ | |
| Pb | Pb2+ | |
| H2 | H+ | |
| Sb | Sb3+ |
No reaction with acids like HCl* |
| Bi | Bi3+ | |
| Cu | Cu2+ | |
| Hg | Hg2+ | |
| Ag | Ag+ | |
| Au | Au3+ | |
| Pt | Pt2+ |
The most active metal is at the top.
*Metals below hydrogen in the table may react with acid if the acid contains an oxidizing anion, as in the case of nitric acid (HNO3).
1Adapted from Wikipedia. (http://en.Wikipedia.org/wiki/Reactivity_series, accessed 10/4/2012)
Activity Series of Metals
In bioinorganic (and inorganic reactions), there are only a few metals that are really useful for redox. Chemists are interested in trying to find the best metals to utilize.
Activity is the tendency of a metal (and hydrogen) to LOSE electrons.
M(s) -> Mn+ + ne-
- Is this an oxidation or a reduction? Explain.
- Predict which is more active, Rb or Sr? Why?
- Predict which is more active, Rb or Cs? Explain.
Metals (in their elemental form) don’t react with each other. So, if one metal is going to lose its electrons, another species (a metal ion) has to pick them up. A typical activity series reaction is the following:
Zn(s) + Cu2+(aq) -> Zn2+(aq) + Cu(s)
- Which species is oxidized in this reaction? Which is reduced?
- What is the oxidizing agent? What is the reducing agent?
- The reaction actually proceeds in the direction written, rather than the reverse. Which metal is more active?
- Propose an experiment that would allow you to determine the relative activity of any two metals.
The ranking of metals (and hydrogen) based on their activity is called the activity series.
Activity Series:
An easy way to use the table is to note that a metal can reduce any ion of a metal below it in the table.
Zinc is more active than nickel, so zinc metal will spontaneously transfer electrons to nickel(II) ions. (Nickel metal will NOT spontaneously transfer electrons to zinc ions.)
According to the table, then, Zn will react to form Zn2+. In order to balance this equation we need to add electrons.
- Complete the two half-reactions, by adding the right number of electrons to the correct side of the reaction arrow.
- Zn -> Zn2+
- Ni2+ -> Ni
- Using the Activity Series Table,
- Predict whether cobalt or copper is more active.
- Draw the half reactions for the reduction of cobalt.
- Draw the half reaction for the reduction of copper.
- Draw the net reaction predicting the direction that the reaction will occur based on the activity series table.
Activity Series vs Periodic Trends
Review: Descriptive chemistry of the alkali metals and alkaline earths.
- As you go left to right in the periodic table, the activity (increases/decrease).
- As you go top to bottom within a column, the activity (increases/decreases).
- Based on periodic trends, the activity of zinc should be (high/low) compared to other transition metals.
- Based on the Activity Series Table, the activity of zinc is (high/low).
Since the experimental data indicates that zinc metal reacts vigorously with acid, there must be more to the activity of zinc than simple periodic trends.
- Write the chemical equation for the oxidation of Zn.
Zinc is defined as reacting vigorously with H+ in aqueous solution. So this is really more than the loss of electrons. There are several steps to get from metal in the solid state to Zn2+(aq).
- For zinc, write the chemical equations for the individual steps:
- sublimation energy (+129):
- first ionization energy(gas) (+906 kJ/mol):
- second ionization energy(gas) (+1733):
- hydration energy (-2046):
- Write the net balanced equation for this reaction that includes all of the steps above.
- The total energy for the process is the sum of the energies of the individual steps. What is the total energy of this process?
Exceptions to the Periodic Trends of Activity: Zn
- Calculate the net energy change for the chemical equation describing the activity of five metals.
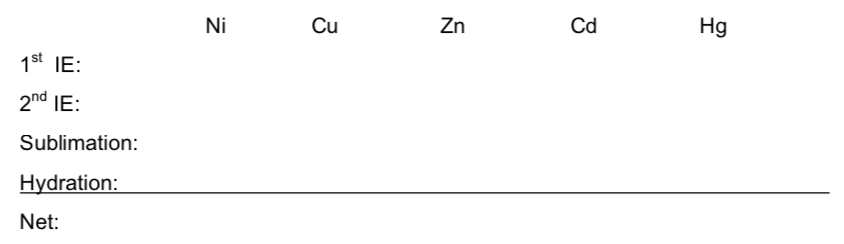
Table of Energy Values for Select Transition Metals
| Ni | Cu | Zn | Cd | Hg | |
| Reduction potential for +2 ion | -0.25 V | +0.34 V | -0.76 V | -0.40 V | +0.85 V |
| 1st and 2nd IE (kJ/mol) and total | 737, 1752 2489 | 746, 1958 2704 | 906, 1733 2639 | 867, 1631 2498 | 1007, 1809 2816 |
| Sublimation energy, for M(s) (kJ/mol) | 375 | 339 | 129 | 113 | 61.3 |
| Hydration energy for the dication (kJ/mol) | -2105 | -2100 | -2046 | -1807 | -1824 |
| Ionic (+2) radius (pm) | 69 | 71 | 74 | 92 |
- Are the net energy values calculated consistent with the order of the reduction potentials for these elements? Which term is the most different when we compare Zn with Cu and Ni?
- State in your own words why zinc is so active compared to these metals.
Exceptions to the Periodic Trends of Activity: Hg
Compare Zn, Cd and Hg. They are in the same family but have dramatically different reduction potentials.
- ΔG is proportional to -E, so a positive value for the reduction potential means that reduction of ions is (spontaneous/nonspontaneous).
You will notice that when the ionization energies of the elements are compared, Hg does not seem to obey the normal periodic trend for this property. One possible explanation for this is something called the lanthanide contraction.
- Use the Periodic Table with radii (in the front section) to compare the radii of 3d transition metal to 4d transition metal.
- How about 4d to 5d?
- Provide an explanation for your observations.
- Explain in your own words why the reduction potentials (or activities) are so different for Zn and Hg.
Exceptions to the Periodic Trends of Activity: Li
If you look at the table of standard reduction potentials, you might see a potential contradiction with what we learned when we looked at the activity series.
- Which alkali metal should be the most active?
- Should this element have the most or least negative value for its standard reduction potential?
Compare Cs+ (-3.02) versus Li+ (-3.04). (Not a big difference but opposite to what we would expect.) We need to compare the relative stabilities of the neutral metal and the related ion.
- Based on your knowledge of ionization energies, which metal should lose an electron more easily? Why?
- In aqueous solution, as metals are being oxidized, what is being reduced?

But the ion that is formed is not in the gas phase! It is in aqueous solution.
- Which alkali cation would bind water molecules more tightly? Why?
- How will this affect the stability of the complex?
- Now draw the potential energy diagram for the reduction of these ions on the same graph.
- Explain why the values of E° might well be close or even reversed from our earlier, less quantitative prediction even though the Cs is more “active”.
Summary of Activity Series
- Define the following terms:
- Activity:
- Reduction Potential
- Complete the following trends:
- As you go left to right in the periodic table, the activity (increases/decrease).
- As you go top to bottom within a column, the activity (increases/decreases).
- Explain the reason for the exceptions:
- Zn:
- Hg:
- Li:
More Practice:
A balanced overall reaction should result when an oxidation half-reaction and a reduction half-reaction are added together. In a balanced reaction, there should be no electrons appearing in the overall reaction. Half-reactions can each be multiplied by appropriate factors so that the number of electrons in the two processes is the same.
- For the following, predict the products.
- Write each half reaction.
- Balance the two reactions to write a complete balanced reaction.
- Predicting the direction that the reaction will occur based on the activity series table
- Sb3+ + Mn ->
- Al + Mg ->
- Cu(NO3)2 + Sn ->
- PbCl2 + FeCl2 ->
- HCl + Ag ->
Application Questions
- Silver nitrate costs about $10/g and is thus a fairly expensive chemical. Waste from a lab experiment contains AgNO3(aq) and Sn(NO3)2 (aq).
- Suggest a feasible method for recovering the silver from the solution (without contamination from Sn metal).
- In the table of the activity series of metals, it is noted that some metals such as gold will not dissolve (react with) in an acid such as HCl but will dissolve in HNO3.
- Calculate the oxidation state of all atoms in the two acids.
- Why is nitric acid considered an oxidizing acid but hydrochloric is not?
- Which category would you think perchloric acid (HClO4) is in?
- A modern “artifact” such as a nail will become almost unrecognizable after being in the ground for a couple of decades, but a Bronze (copper and tin alloy) Age implement can be identified after more than a thousand years in the ground.
- Explain the difference.
- Descriptive Chemistry Project
- Research your element (one that you have chosen or been assigned by your instructor) using print or online sources.
- Write a 1-2 paragraph summary containing key information about your element -- its discovery and isolation, physical description, chemical and physical properties, etc. You may also wish to emphasize properties that are typical as well as the unusual.
- Submit two good “trivia questions” about your element. A good trivia question is not overly specific, not too easy, but teaches or emphasizes a key aspect of your element. Also submit your answers. You should also assume that the person answering your question will have access to a periodic table.
- Examples of bad trivia questions: “This element has the seventh highest boiling point.” “What is the boiling point of argon?” “What is the chemical symbol (or atomic weight) for sodium?”
- Good trivia questions: “ At 4 K, the boiling point of this element is the lowest of all elements.” “This silver main group metal forms +1 and +3 oxidation states of comparable stability and is used a emiconductor dopant.”
- Your instructor will collect and make available all students’ info about their elements.
- The best trivia questions will be used in a trivia contest. Valuable prizes or bonus points may be awarded. Furthermore, some of the best trivia questions might be used on an upcoming exam or PSA.


