29: Olefin Metathesis
- Page ID
- 151240
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\dsum}{\displaystyle\sum\limits} \)
\( \newcommand{\dint}{\displaystyle\int\limits} \)
\( \newcommand{\dlim}{\displaystyle\lim\limits} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\(\newcommand{\longvect}{\overrightarrow}\)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)Name: ______________________________
Section: _____________________________
Student ID#:__________________________
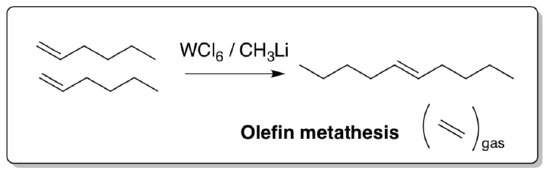
Olefin Metathesis
In olefin (or alkene) metathesis, a C=C bond is split in half and reattached with another partner.
Olefin Metathesis: Overall Reaction
Yves Chauvin, Dick Schrock and Bob Grubbs shared the 2005 Nobel Prize in Chemistry for their work in this area.
- Show the products of the following olefin metatheses:
Olefin Metathesis: Control
If you look at these reactions, you will notice that the products could also react using the same mechanism to return to the starting materials.
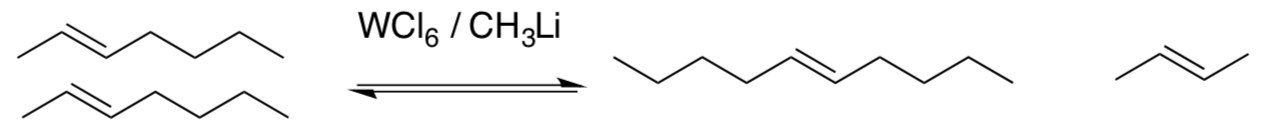
The reaction shown above produces mostly products (right).
- How is this driven forward?? Hint Le Chatelier
Ring-closing metatheses are usually accomplished using Grubbs’ catalysts (examples shown to the right).
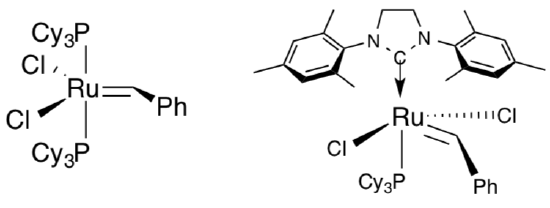
- RCM reactions using Grubbs’ catalysts are usually run dilute. Propose a reason.
Polymer reactions usually use Schrock’s catalyst (example shown to the right).
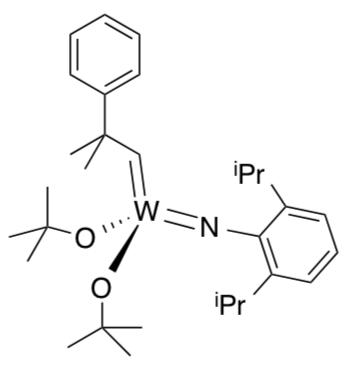
- Polymer reactions using Schrock catalysts are usually run neat. Propose a reason.
Schrock catalyst is orders of magnitude faster than Grubbs’ catalyst.
- Why would that be advantageous if you want to make polymers?
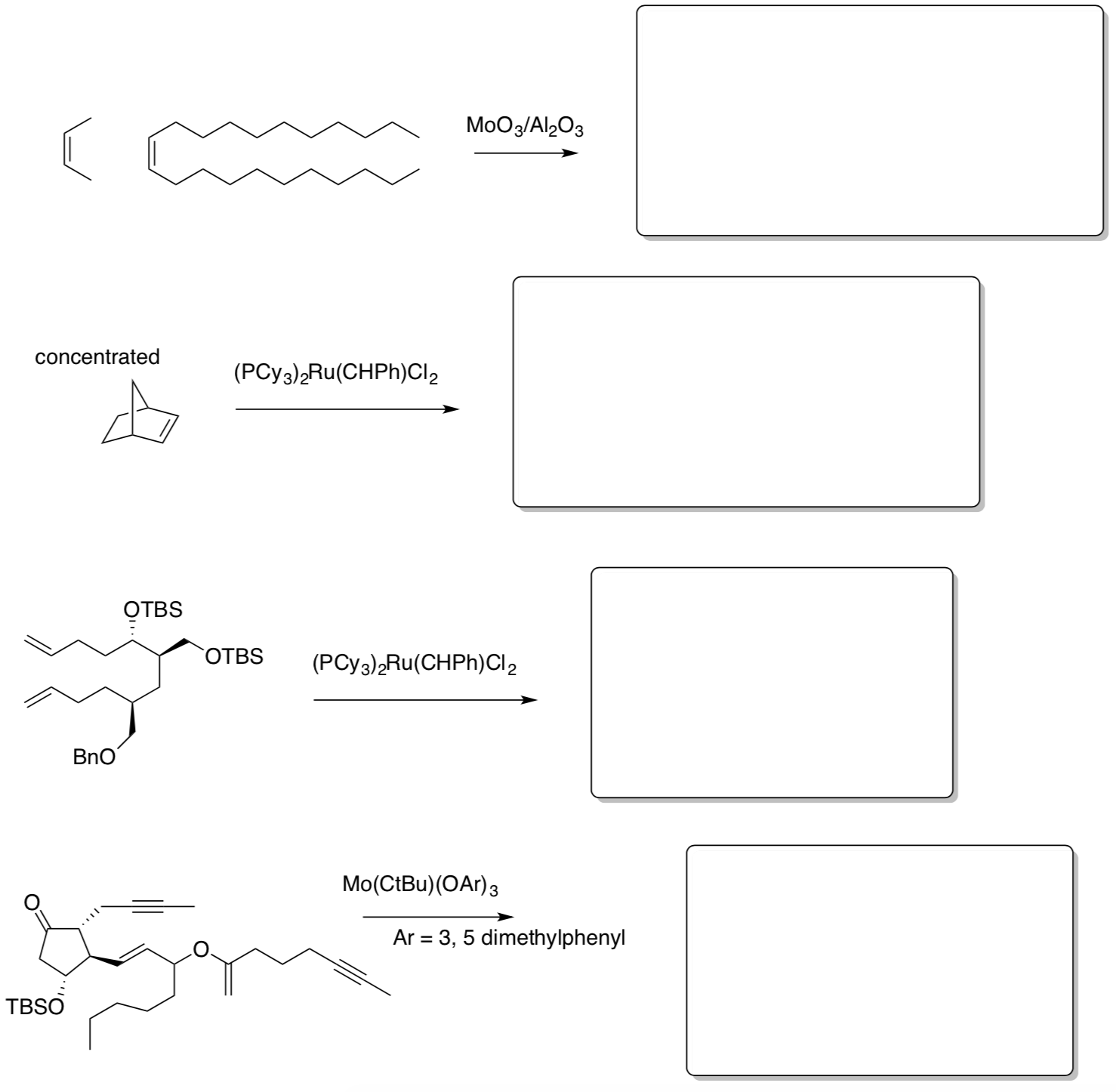
Olefin Metathesis: Practice
- Fill in the products of the following reactions.
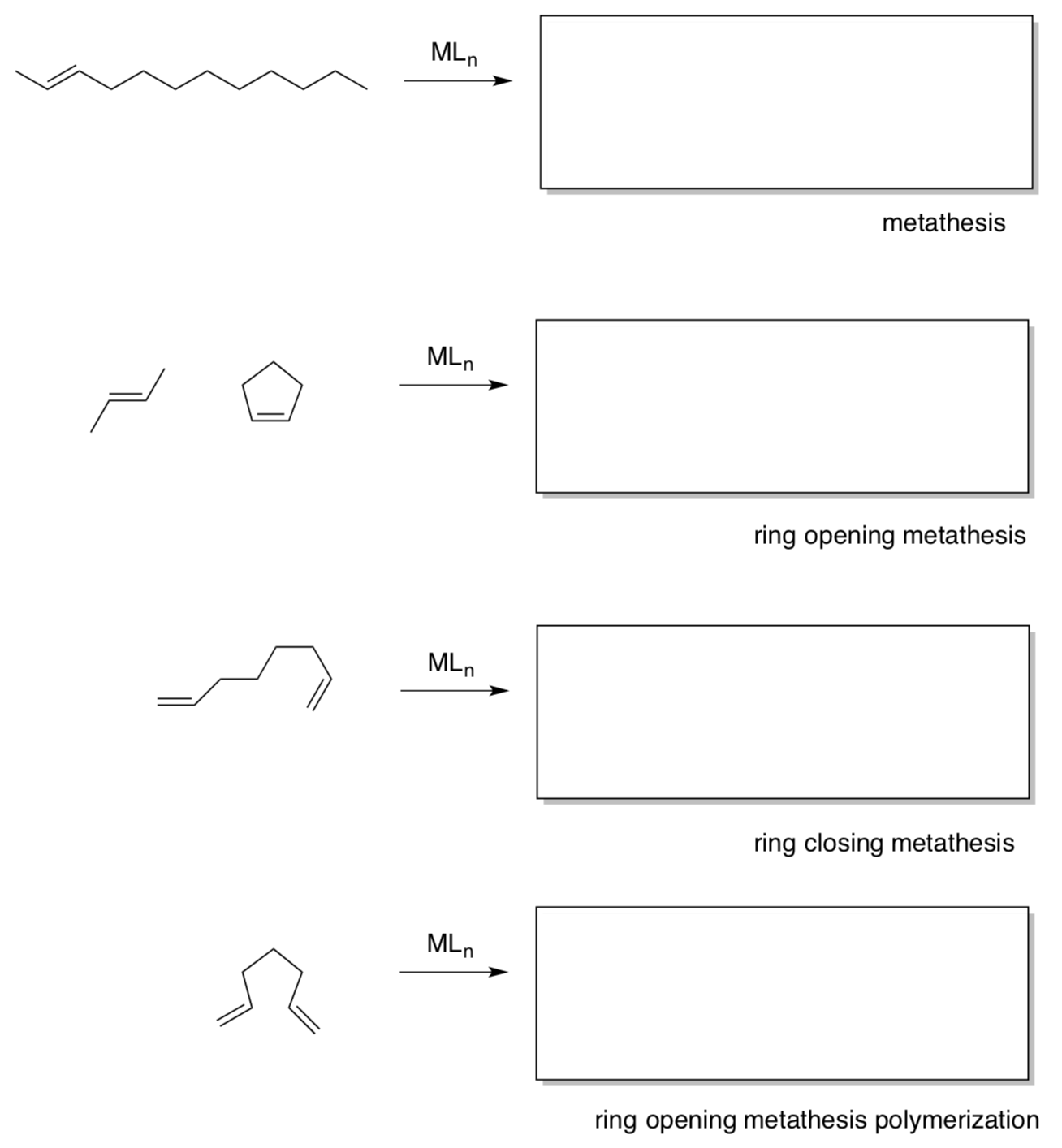
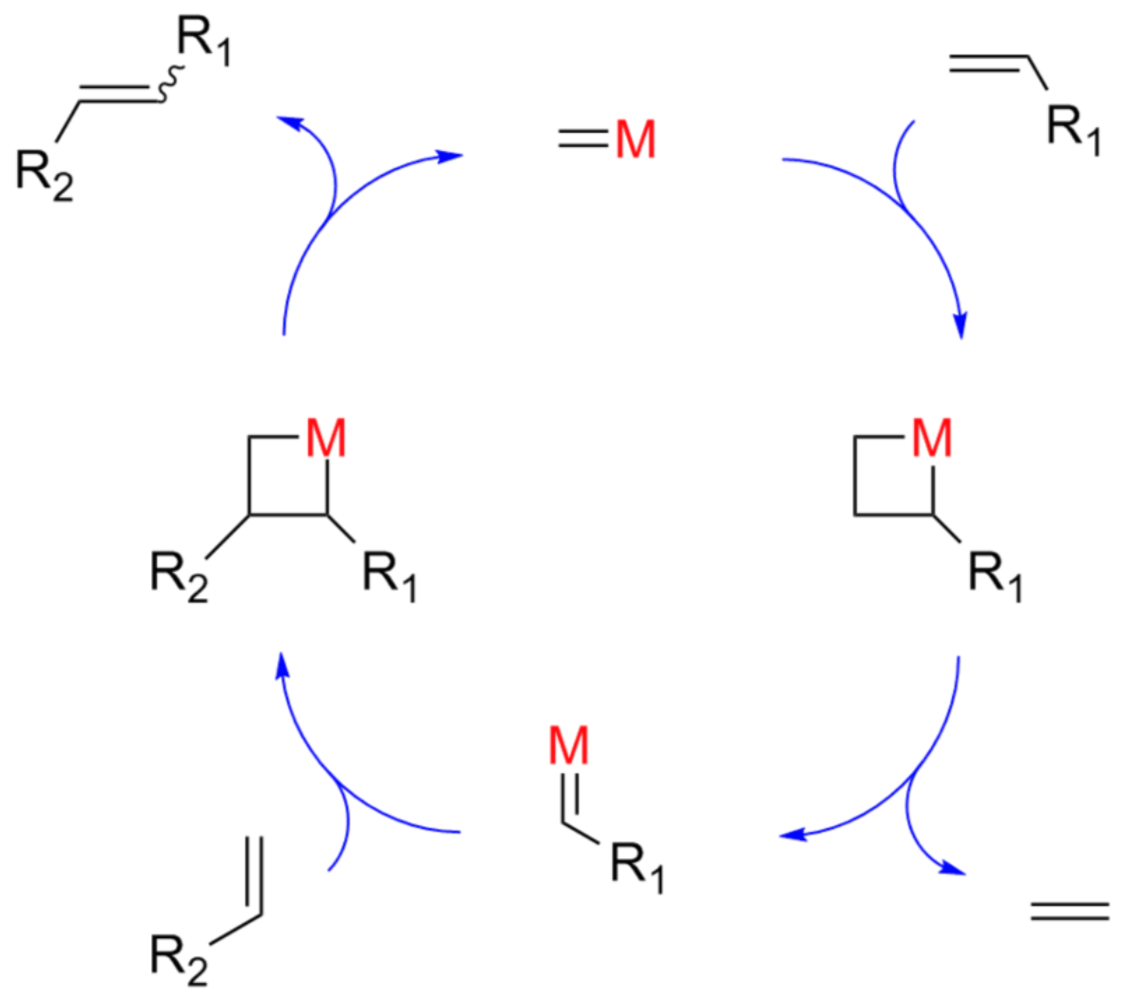
Olefin Metathesis: Introduction to the Mechanism
Chauvin first proposed a mechanism of transition metal alkene metathesis.
- Predict the product for this mechanism.
- Draw the arrows for the steps in this mechanism:
Olefin Metathesis: Mechanism Determination
Chauvin was working as a bachelor’s-level chemist at the French Petroleum Institute in the 1960’s. He was an avid reader of the chemical literature, and these two reports caught his eye:
- Fill in the products of the olefin metathesis reactions.
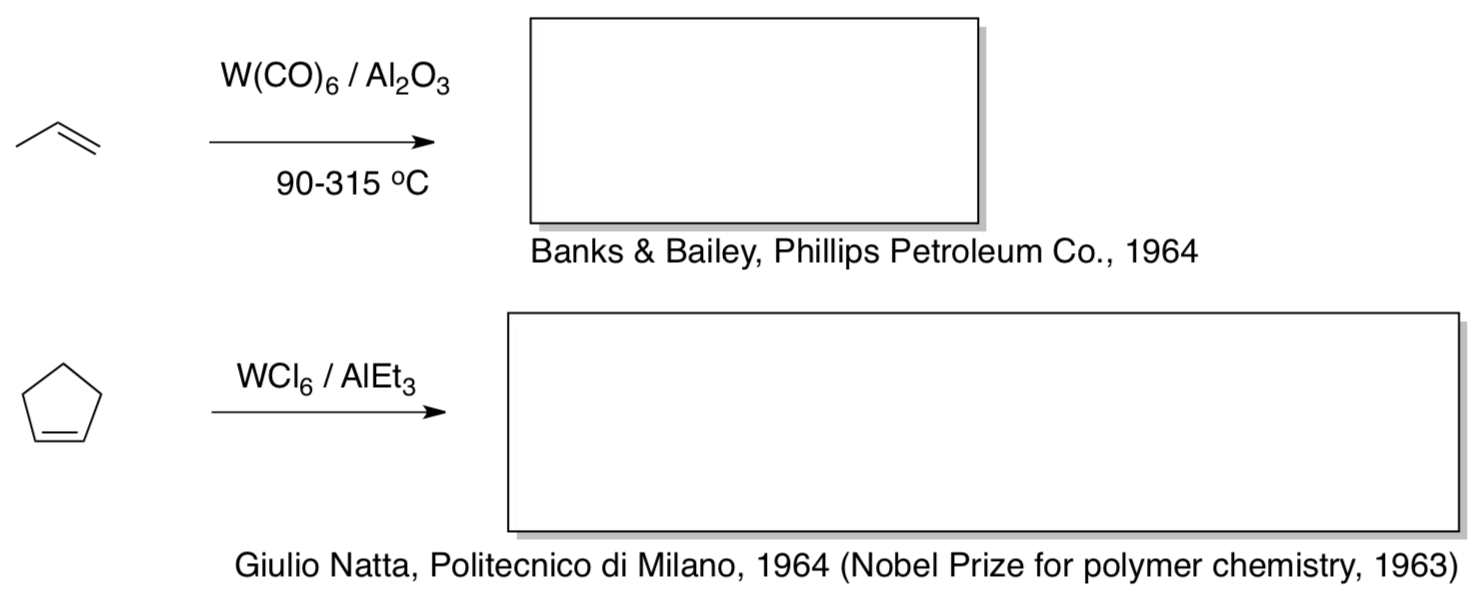
Chauvin felt sure that these developments were related to another paper he read that year...
Olefin Metathesis: Carbene Complexes
In 1964, E.O. Fischer at the Technical University of Munich first reported the existence of a metal-carbon double bond. For this and other developments, he received the Nobel Prize in Chemistry in 1973.
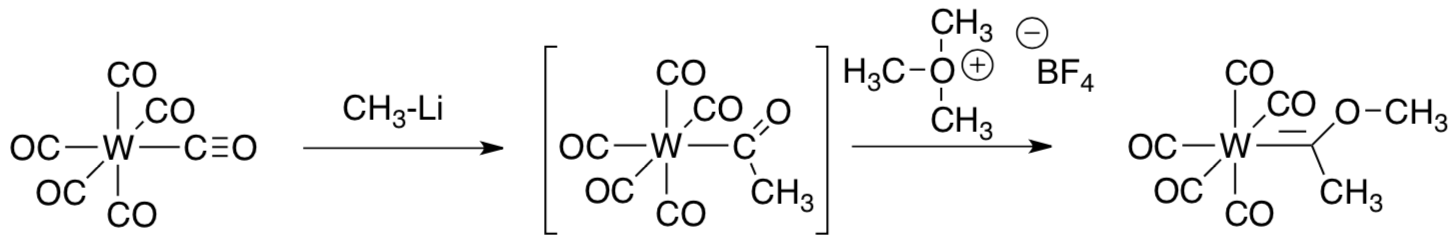
- Show a mechanism for formation of this “carbene complex” using curved arrows.
For some time, these bonds were only observed if a heteroatom was next to the “carbene” carbon, such as the oxygen in Fischer’s case.
- Show, with an MO cartoon, why the heteroatom might stabilize the carbene.
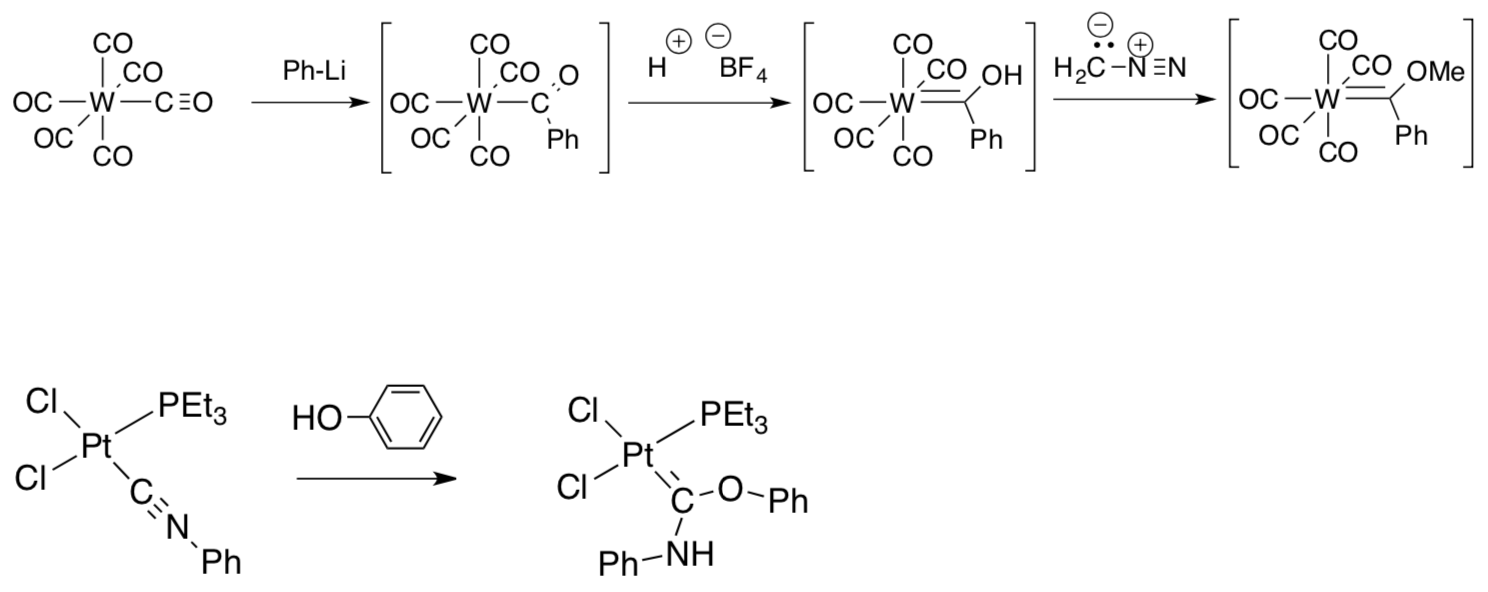
- Provide mechanistic arrows (and missing intermediates) for the following variations:
Olefin Metathesis: Chauvin’s Mechanism
Thinking that all three of these developments were related, Chauvin first set out to see whether metathesis and polymerization might both happen under the same conditions. He observed the following products (plus starting materials, and two sets of longer chains, C19-C21 and C24-C26) after 15 minutes.
- Draw the products.
- Show where the products came from, via olefin metathesis.

- Why are some compounds, such as C12H22, not formed?
- Why is the middle member of each trio found in higher amounts?
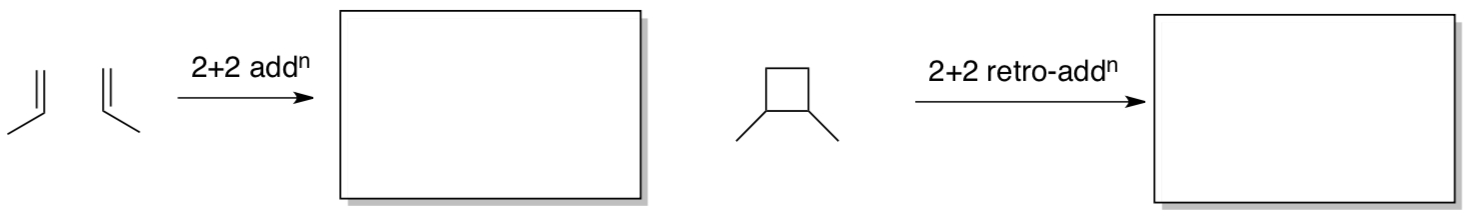
Chauvin suspected the reaction might involve 2+2 addition to an M=C group, like those in Fischer’s carbenes, followed by 2+2 retro-addition.
- Show the possible product(s) of the reactions below.
Olefin Metathesis: Chauvin’s Mechanism
To see whether a metal-ligand bond might be participating in the metathesis, Chauvin tried the following experiment:
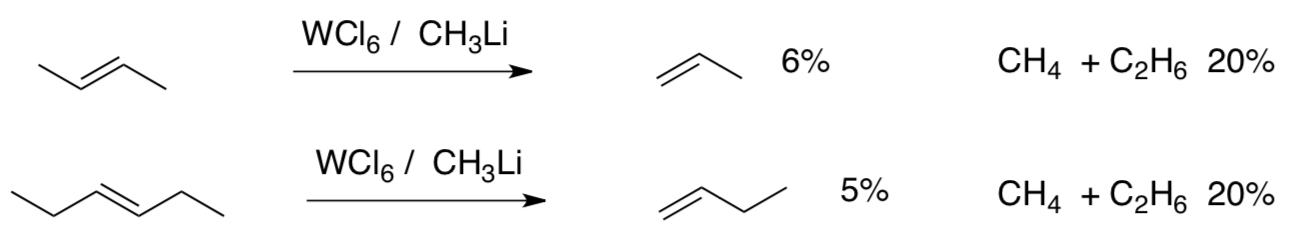
- Draw a line through each starting alkene, showing where it would be split in a metathesis.
- Draw a line through the alkene products, showing where they would be knit together during metathesis.
- Why did Chauvin select CH3Li as an alkylating agent, rather than CH3CH2Li or CH3CH2CH2Li?
- Draw a possible metal carbene that may have participated in this reaction.
- Show its 2+2 addition and the subsequent 2+2 retro-addition to form one of the products above.
- Propose mechanisms for formation of (a) methane and (b) ethane.
Chauvin’s proposal was not met with universal enthusiasm. One problem was the rules of pericyclic reactions.
- Draw
- the Huckel MO diagram for ethene;
- a cartoon of the LUMO of ethene;
- a cartoon of the HOMO of ethene.
- What is wrong with the HOMO-LUMO interaction in a 2+2 addition?
- What is wrong with the metal carbene proposed by Chauvin?
Olefin Metathesis: Carbene Complexes
In 1972, Dick Schrock joined DuPont as a Ph.D. chemist and was asked to develop new areas of chemistry that might be of long-term use to the company. He soon reported the observation of an “alkylidene”.
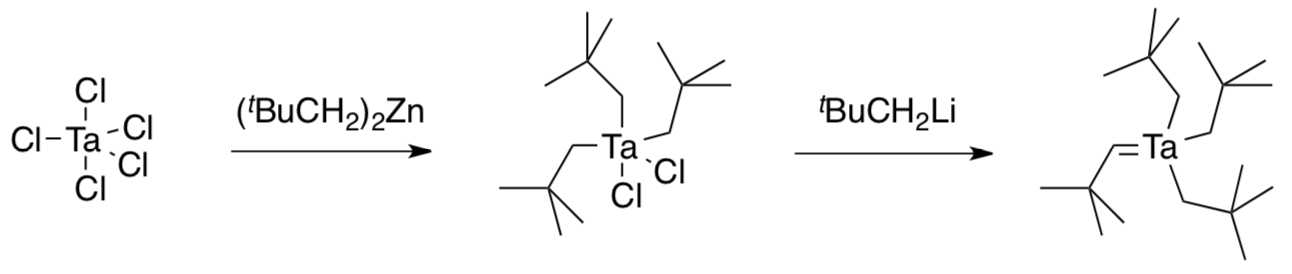
- How did this report help Chauvin’s case?
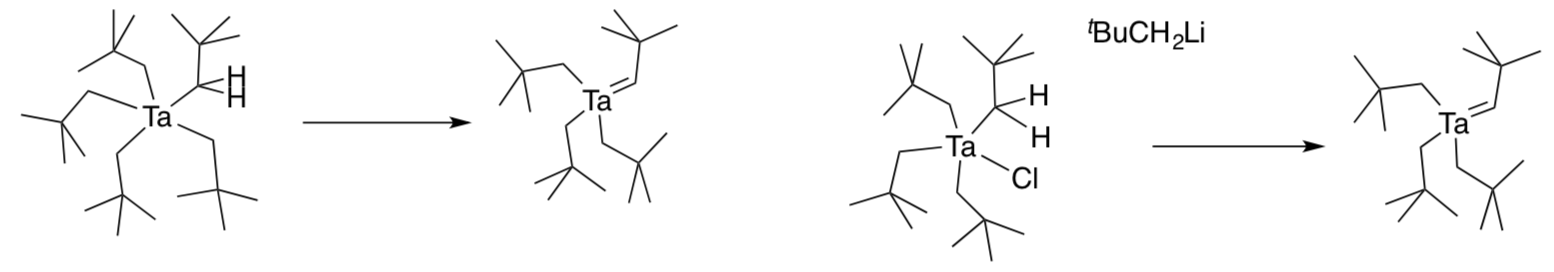
Schrock postulated that this compound could form in two possible ways.
- Show both mechanisms with curved arrows.
- 1,2-elimination of HCl from (tBuCH2)4TaCl with a bulky base
- “alpha”-elimination, in which an alkyl group deprotonates its neighbor as it leaves from (tBuCH2)5Ta.
- In what percent of product would you expect to find deuterium on the carbene carbon if (tBuCH2)3TaCl2 were reacted with deuterium-labeled tBuCD2Li via mechanism (a)?
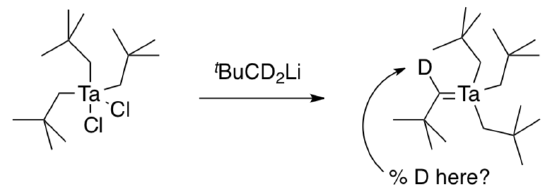
- via mechanism (b)?
- Schrock observed 38% of the carbene positions deuterated. Which mechanism probably occurs?
Olefin Metathesis: Making Better Catalysts on the East Coast
By 1980, Schrock had moved from DuPont to MIT, where his lab was developing better metathesis catalysts.
- Propose reagents for each step in the synthesis.
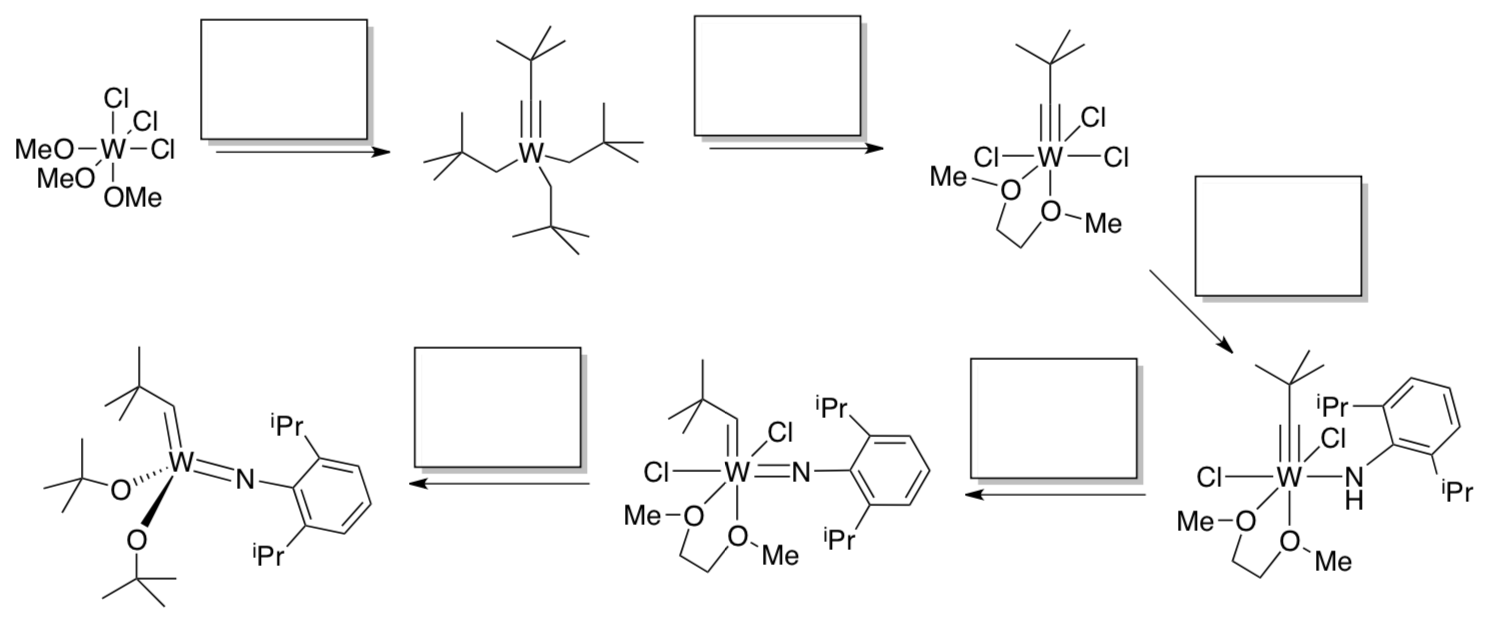
Compounds of this type (lower left) are effective metathesis catalysts. The analogous molybdenum compounds are among the fastest metathesis catalysts; options are available that offer excellent control over cis vs. trans bond formation.
Similar catalysts were developed for alkyne metathesis.
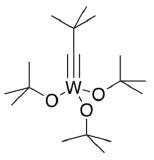
- Propose a synthesis of the catalyst from WCl6.
- Complete the following synthesis of the perfume, civetone.
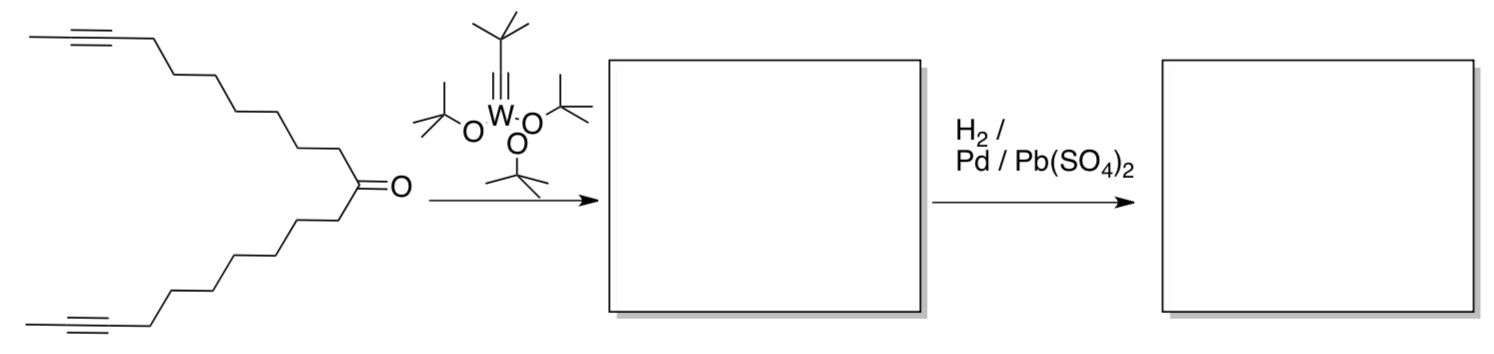
- Complete the following synthesis of the perfume, civetone.
Olefin Metathesis: Making Better Catalysts on the West Coast
Meanwhile, Bob Grubbs at CalTech sought metathesis catalysts that were more tolerant of air, moisture and oxygen/nitrogen-containing functional groups.
- Classify in terms of hard/soft acid/base

- Explain briefly why unwanted hard/soft acid/base interactions may interfere with catalysis.
The new catalyst was based on a precursor, below right, from Geoff Wilkinson’s lab at Imperial College. Wilkinson shared Fischer’s 1973 Nobel Prize.
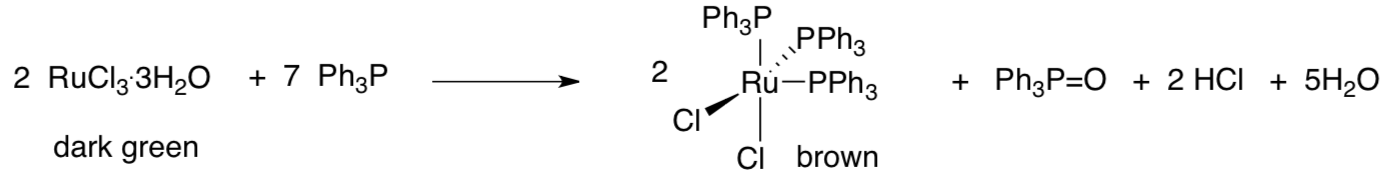
- What is the oxidation state of ruthenium (a) before and (b) after the reaction?
- What is the electron count on ruthenium in the brown dichloride complex? Show your work.
- Triphenylphosphine is a new ligand in the reaction. What is its other role?
The first generation of catalysts from the Grubbs lab are stable to air and water. Their ease of use quickly made them the preferred catalysts worldwide for routine metatheses.
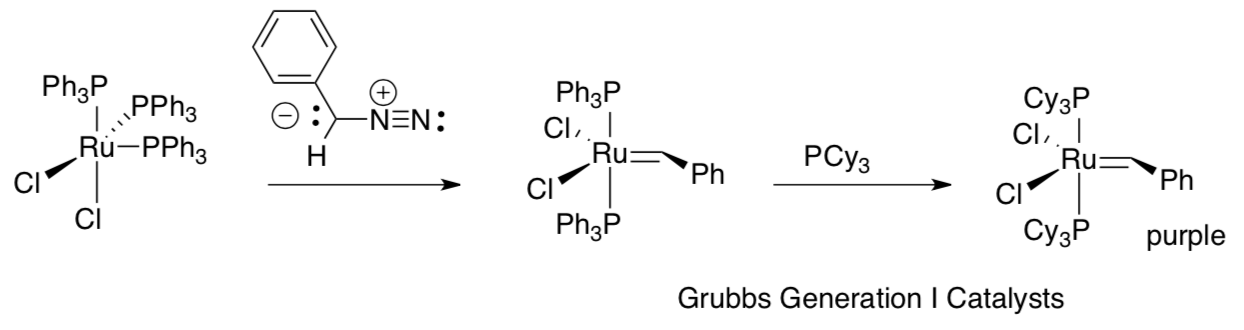
Olefin Metathesis: Making Better Catalysts on the West Coast
- Provide a mechanism for the formation of metal carbene in the above synthesis.
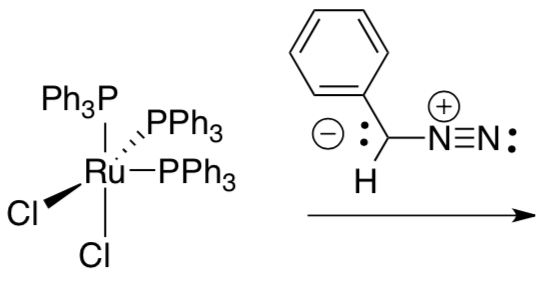
Replacement of triphenylphosphine (PPh3) with tricyclohexylphosphine (PCy3) resulted in a significant rate increase; a strong sigma-donor seems to be crucial.
- Draw PPh3 and PCy3 and explain why the latter is more electron donating.
Kinetics work in the Grubbs lab shed light on the mechanism of metathesis with tis catalyst. For example, the reaction showed the following dependence on added phosphine:
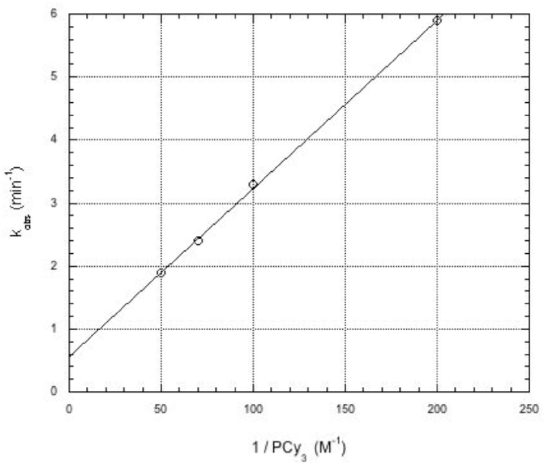
- What happens to rate (kobs) when phosphine is added?
- This “inverse order” typically means phosphine is produced in an equilibrium during the reaction. Show this equilibrium.
Olefin Metathesis: Making Better Catalysts on the West Coast
Further support for loss of a phosphine during metathesis came from the observation of crystals during the following reaction by a group at Boston College, allowing x-ray analysis. (Snapper, J. Am. Chem. Soc. 1997, 119, 7157- 7158)
- Show the product of the first metathesis reaction (addition and retro- addition) between these reactants.
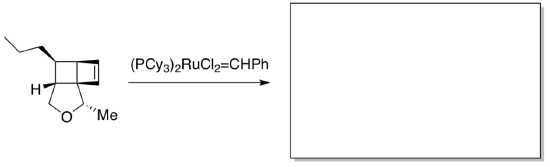
- In the crystal structure, put a circle around ruthenium, triangles around the chlorides, an ellipse around the PCy3, a rectangle around the metal carbene and a heart around the coordinated alkene.
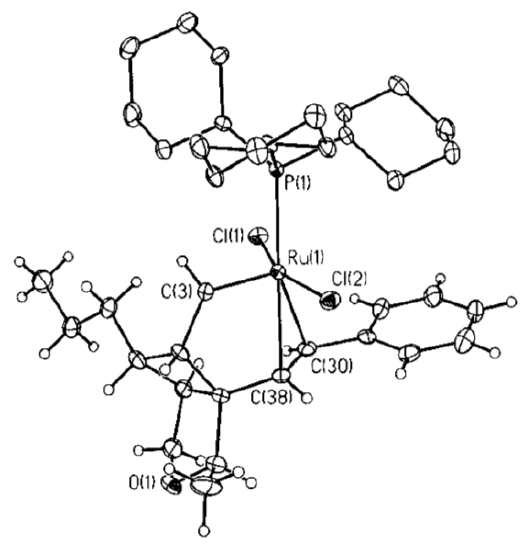
- What is missing?
- Why might loss of one of the phosphines be important for catalysis?
Kinetics studies also showed that, at very high levels of alkene, the rate of reaction actually decreases.
- Propose a reason for the rate decrease. (Probably related to the previous observations; remember, the phosphine also plays a role as a strong sigma donor, tuning up the reactivity of the complex.)
Olefin Metathesis: Making Even Better Catalysts on the West Coast
The Grubbs lab decided to replace one of the phosphines with a more strongly sigma-donating “N-heterocyclic carbene”.
- Show the resulting catalyst.
- How might this stronger donor prevent deactivation at high alkene concentrations?
- How might this stronger donor promote alkene binding (think about trans effect)?
Olefin Metathesis: Application
- Shell Higher Olefin Process
One application of olefin metathesis is found in the Shell Higher Olefin Process (SHOP), which supplies raw materials for making soaps (sodium lauryl sulfate, etc).
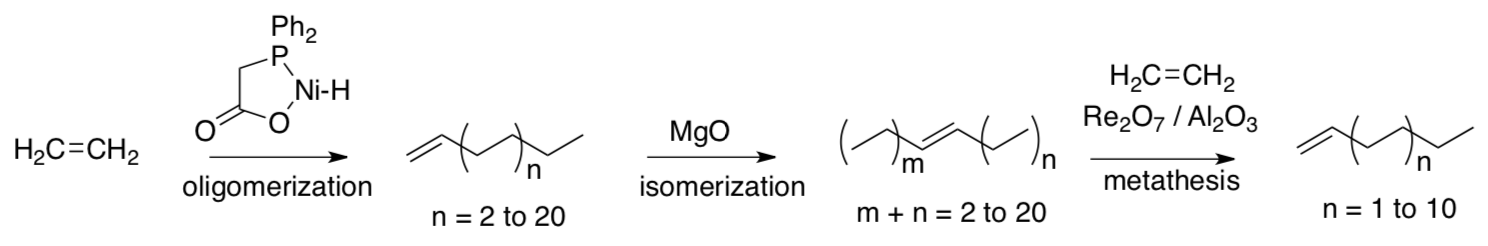
- Review: Provide a mechanism for the oligomerization step (1,2 insertions).
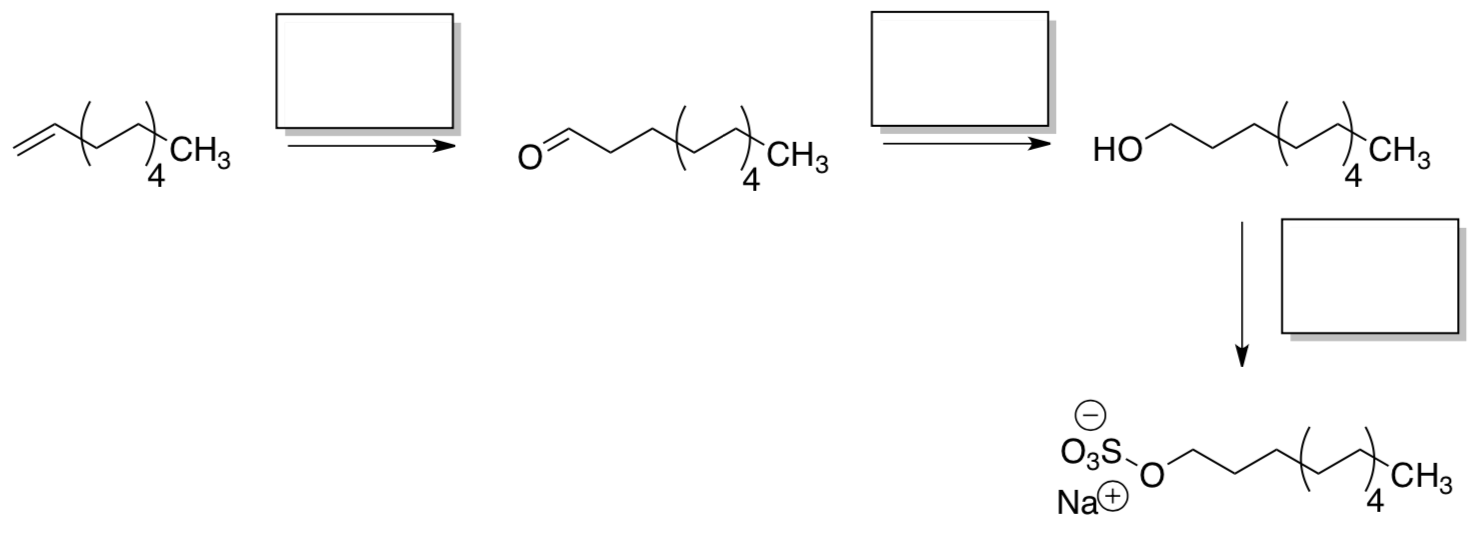
SHOP allows the statistical distribution of olefins produced in oligomerization to be adjusted and optimized. The desired fractions are then subject to hydroformylation, reduction, and sulfonation.
- Suggest reagents to complete the preparation of sodium lauryl sulfate.
- Professor Hoye and others (Organic Letters, 1999, 2, 277-279) were looking at synthesizing a natural product (±)-Differolide (compound 1).
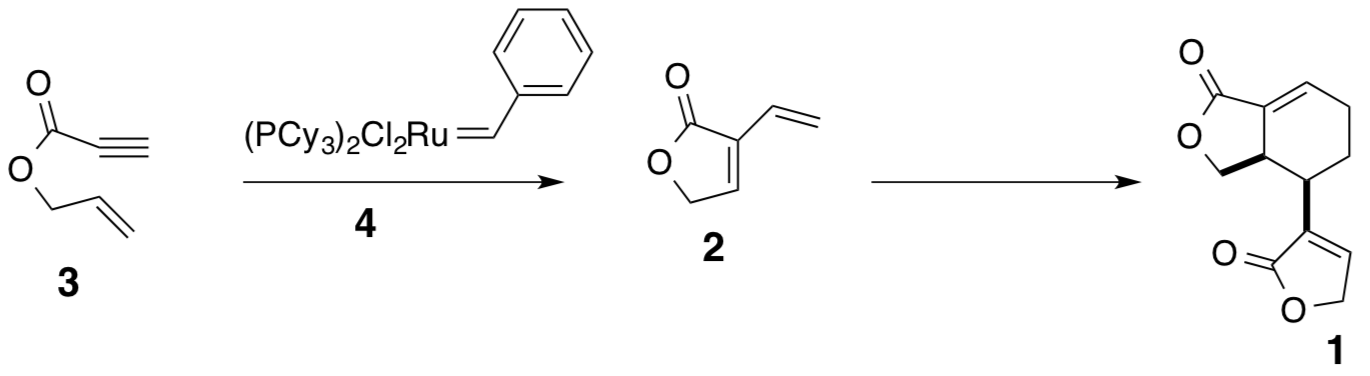
- The first step is an olefin metathesis mechanism. It is postulated that it can occur two different ways (“yne-then-ene” or “ene-then-yne”). Evidence for the “ene-then-yne” mechanism is the production of an aromatic side product. Propose a mechanism (catalytic cycle) for this “ene-then-yne” reaction. Hint: Compound 4 is an initiator and two molecules of compound 3 are needed to generate compound 2.
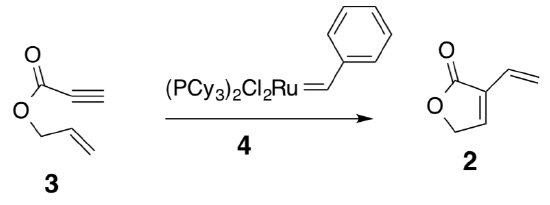
- What is the structure of the aromatic side product that is produced from this reaction?
- Compound 4 is referred to as a carbene complex. Draw a resonance structure of 4 and explain how it is a carbene.
- The last step in this mechanism to produce compound 1 is a dimerization of compound 2. Propose a mechanism for this last step.
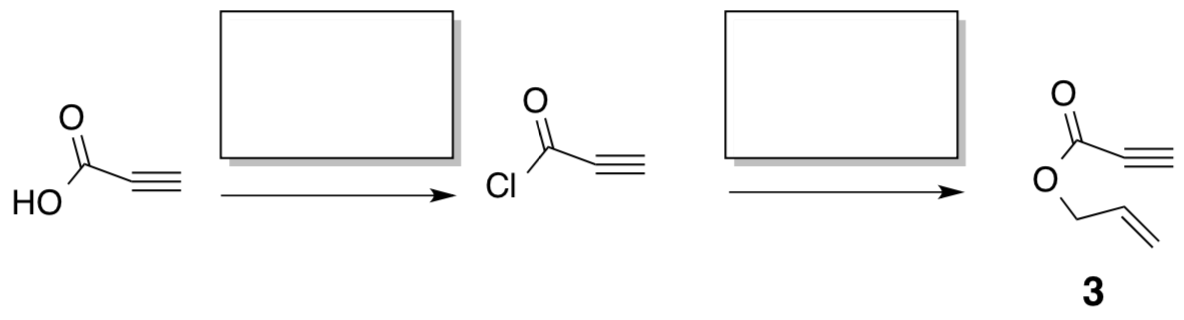
- Compound 3 can be synthesized as outlined below. Provide reagents for the steps.
- The first step is an olefin metathesis mechanism. It is postulated that it can occur two different ways (“yne-then-ene” or “ene-then-yne”). Evidence for the “ene-then-yne” mechanism is the production of an aromatic side product. Propose a mechanism (catalytic cycle) for this “ene-then-yne” reaction. Hint: Compound 4 is an initiator and two molecules of compound 3 are needed to generate compound 2.
- Photoactivation of Ruthenium Olefin Metathesis Initiators
Ben-Asuly, Aharoni, Diesendruck, Vidavsky, Goldberg, Straub and Lemcoff,Organometallics 2009, 28, 4652-4655.A report on a latent metathesis precatalyst that may be activated by the use of light irradiation and may be returned to the off state by heat.
- The following inactive precatalyst was prepared. What is the geometry and electron count of the complex?

Valence electrons on Metal:
Charge on the ligands:
Charge on the Metal:
Revised Count on the Metal (accounting for charge): Number of electrons donated from the ligands:Total electrons in this complex:
- Why is this complex likely to be inert as a complex?
- Which is the weakest binding ligand? Explain your choice.
- Which ligand will dissociate when this complex is irradiated with visible light?
- Complete the following scheme for the conversion of the pre-catalyst to the catalyst and back to the pre-catalyst.
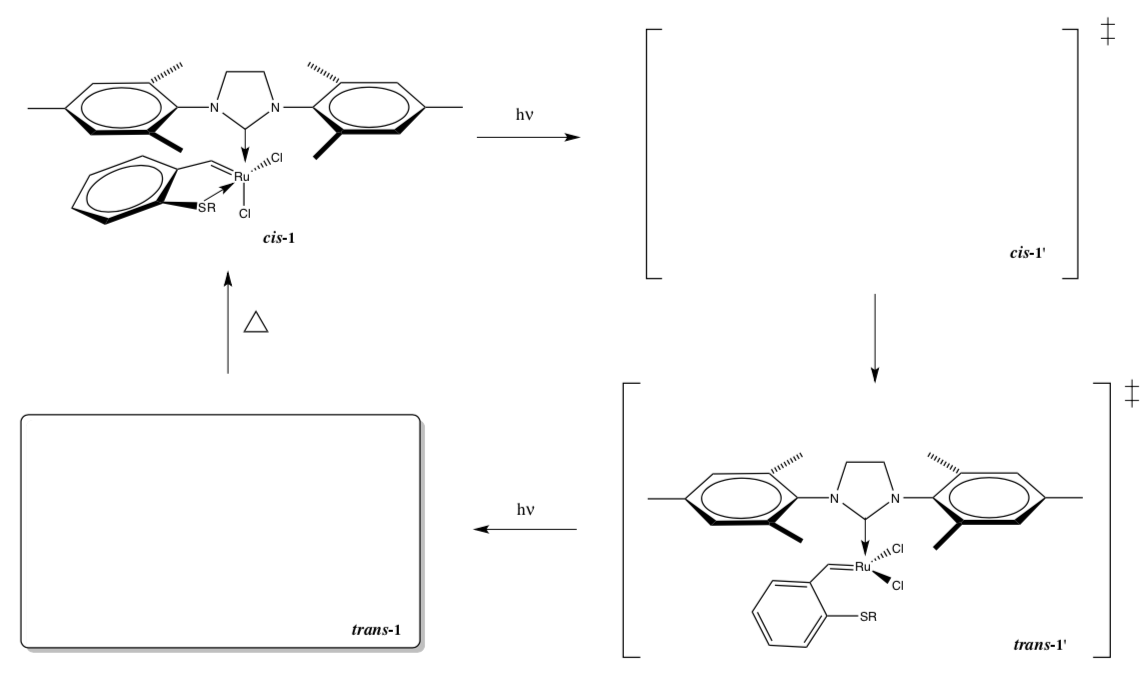
- Which of the structures above is likely the active catalyst?
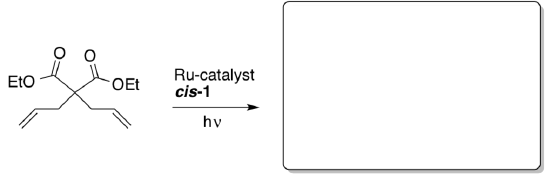
- When the catalyst is active it is capable of catalyzing the following metathesis reaction. Predict the product.
- Why does heating the Ru-trans-1 complex drive it back to the Ru-cis-1 structure?
- The following inactive precatalyst was prepared. What is the geometry and electron count of the complex?


