26: Pericyclic
- Page ID
- 150983
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)Name: ______________________________
Section: _____________________________
Student ID#:__________________________
Template:HideTOCIntroduction to Pericyclic Reactions
A pericyclic reaction is a type of concerted reaction wherein the transition state of the molecule has a cyclic geometry.
Simple Mechanism
The mechanism for a pericyclic reaction
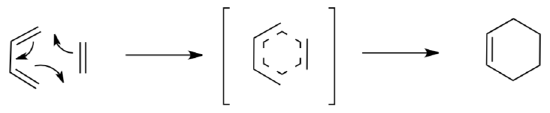
- How many electrons are moving through aligned p orbitals during the transition state?
- By Huckel’s Rule, how would you characterize the transition state?

Because these reactions proceed with no cationic, anionic or radical intermediates, chemists struggled to understand the mechanisms.
- Draw a Reaction Progress Diagram for a concerted reaction.
- Based on this information, draw the products for the reactions below.
Introduction to MO of Cycloadditions
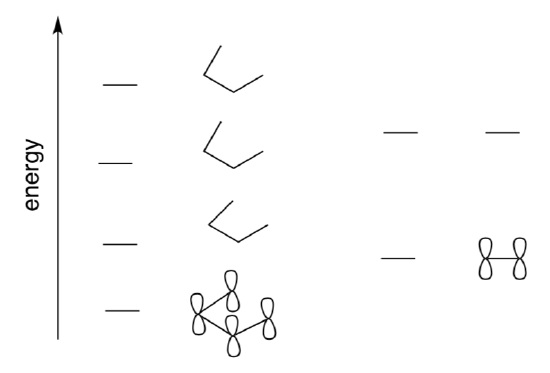
In 1965, R. B. Woodward and Roald Hoffman on the mechanism on the MO of pericyclic reactions which resulted in a Nobel Prize. They posited that bonding overlap must be maintained between orbitals during the course of a concerted pericyclic reaction.
Cycloadditions involve the interaction of HOMO of one molecule with LUMO on the other.
- Add the p orbitals to illustrate the Huckel MO diagrams of butadiene and ethene.
- Circle the HOMO in butadiene and the LUMO in ethene.
- Looking at the LUMO of the ethene and the HOMO of butadiene interactions, explain how the sigma bonds are formed. Why does the ethene need to approach from below?
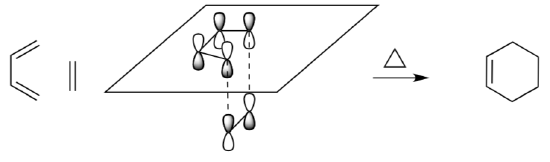
Other cycloadditions are not so successful.

- Draw the LUMO of ethene and the HOMO of ethene to illustrate a potential bonding interaction.
- What is wrong?
Geometry, Regiochemistry, and Stereochemistry of Diels-Alder [4+2] Reactions
Diels Alder Diene Structural Considerations
1,3-butadiene can adopt two stable conformations:
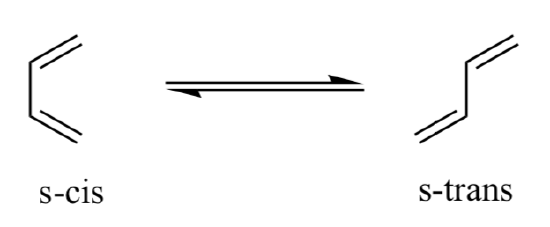
- Which conformation do you predict is more stable?
- Which conformation do you predict is necessary for Diels Alder reaction to take place?
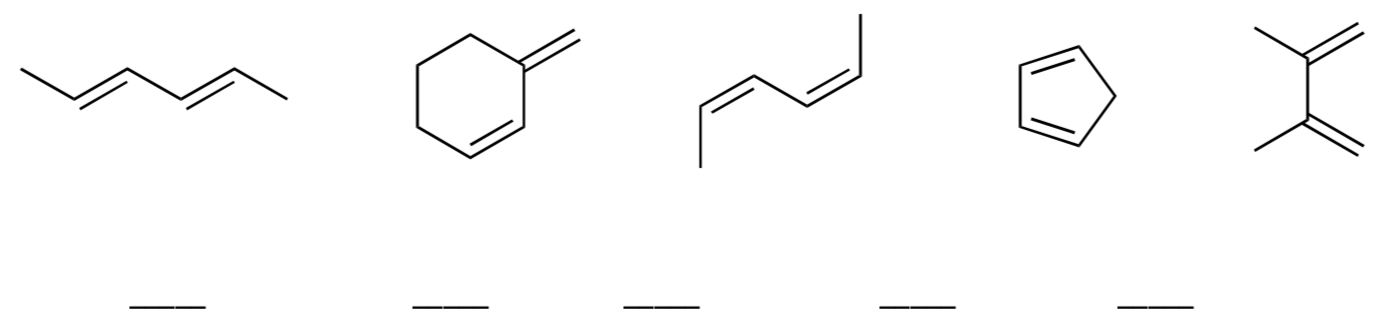
- Rank the following dienes in terms of how quickly they will react in a Diels Alder reaction (1 = fastest)
Diels Alder Regiochemistry
Sometimes, products with different regiochemistry are possible depending on the orientation of the two reactants.
- Draw the two possible isomers from this reaction.
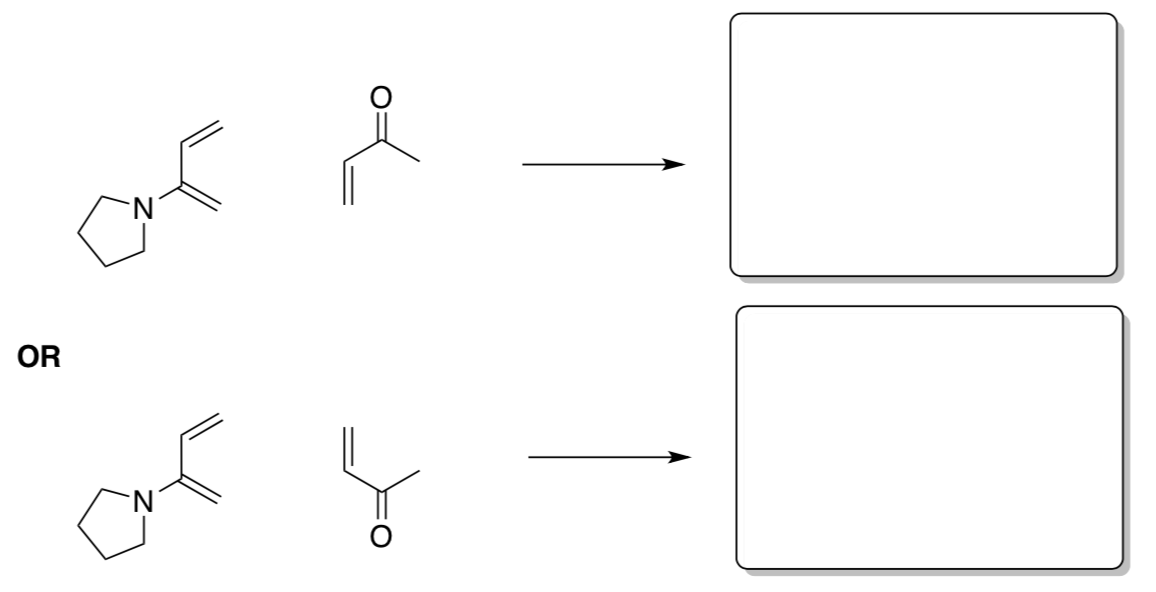
Sometimes, cycloadditions are guided by polarity.
- Draw the resonance structures that illustrate that this diene is really a nucleophile and this dienophile is really an electrophile.

- In the reactions above, indicate which is the preferred product.
- Draw the preferred products in the following cases.
Diels Alder Stereochemistry
- Explain why the transition-state illustrated below (MO Picture) shows an allowed reaction pathway.
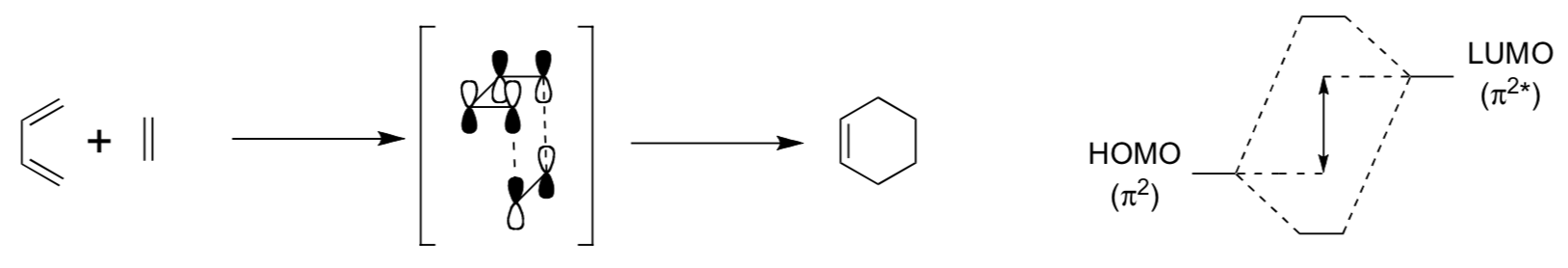
- The reaction shown below is diastereoselective, but not enantioselective, why?
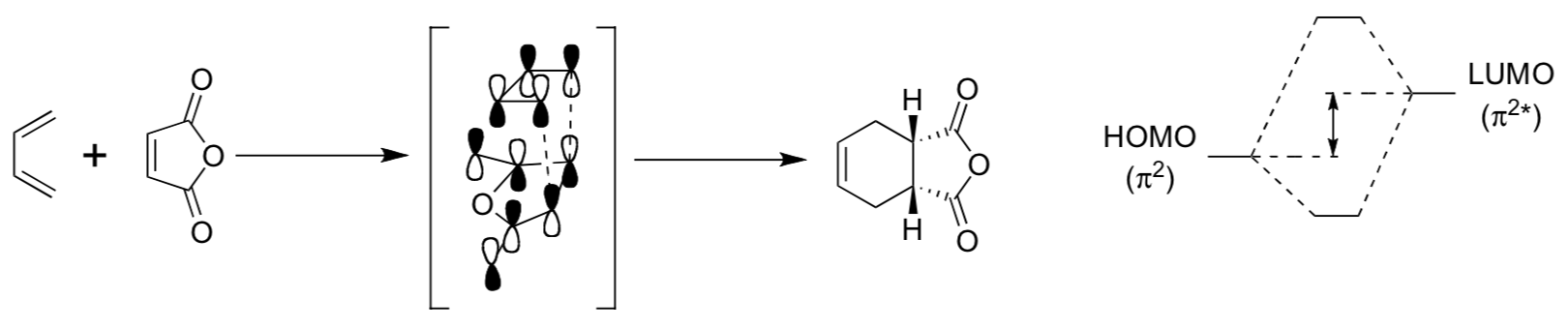
- The reaction shown below is diastereoselective, but not enantioselective. Why?
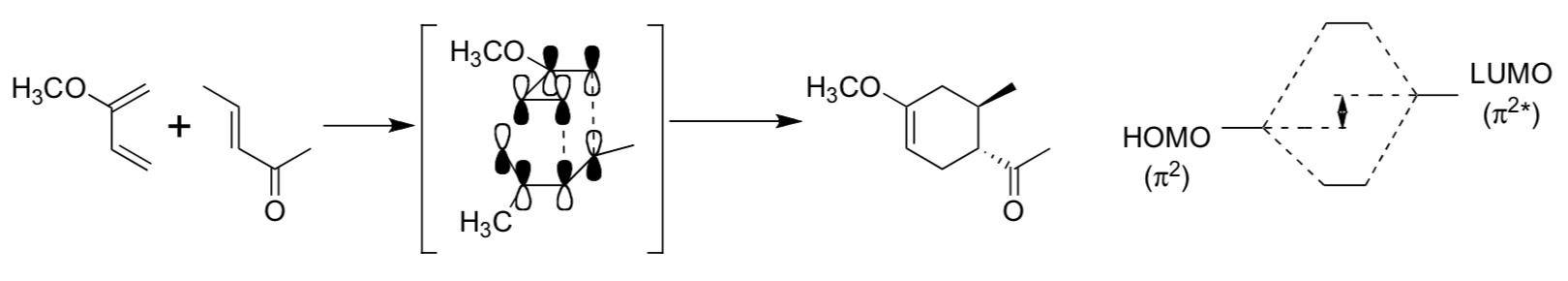
- Using the MO mixing diagrams for each reaction above, explain why DE (LUMO- HOMO) decreases in each successive example.
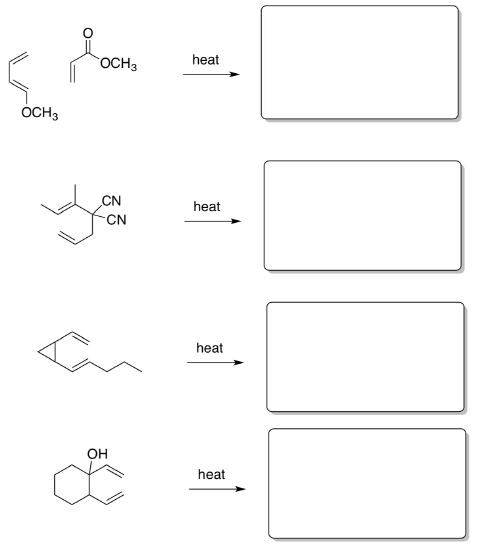
Practice Problems:
- Show the products of these reactions including regiochemistry:
- Show the reagents needed to prepare these products through Diels-Alder Reactions.
Molecular Orbital Theory of Pericyclic Reactions
Woodward and Hoffman developed rules to predict whether pericyclic reactions would occur. The rule is based on the idea that the highest energy electrons will be involved in the movement.
Note
In intramolecular reactions, look at the HOMO.
In the cycloaddition reactions, look at the HOMO of one and the LUMO of the other.
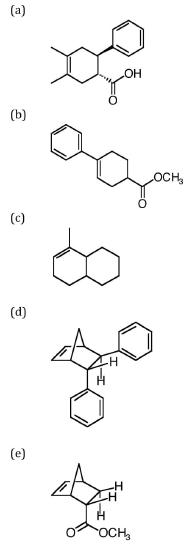
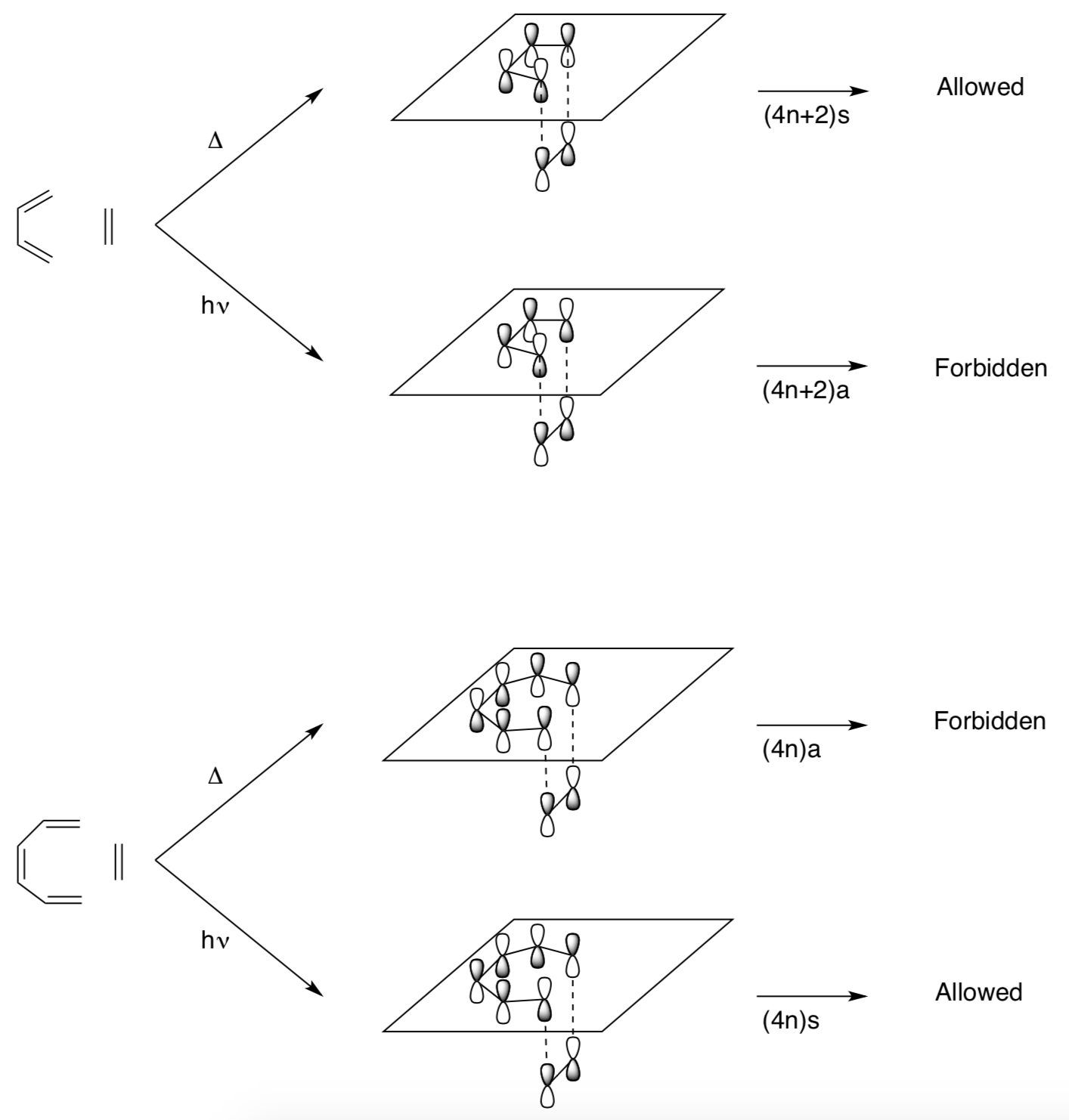
Simplified Woodward-Hoffman Rule #1
A pericyclic reaction involving 4n+2 electrons is thermally allowed if all of the components bond to the same face (suprafacial).
- Which of the following reactions would be likely to occur based on this rule?
Simplified Woodward-Hoffman Rule #2
A pericyclic reaction involving 4n electrons is thermally allowed if the orbitals add from opposite faces (antarafacial).
- Which of the following reactions would be likely to occur based on this rule?
Photocatalysis?
We have seen that [2 +2] reactions do not occur because there is no in-phase overlap the orbitals.
However, they may occur under different conditions.

- What does the HOMO (SOMO) of ethene look like after excitation with light?
- Looking at the LUMO of ethene and the HOMO of ethene* to illustrate a potential bonding interaction.
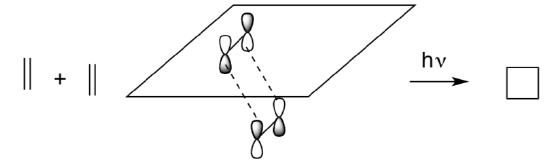
- Why does it work under these conditions?
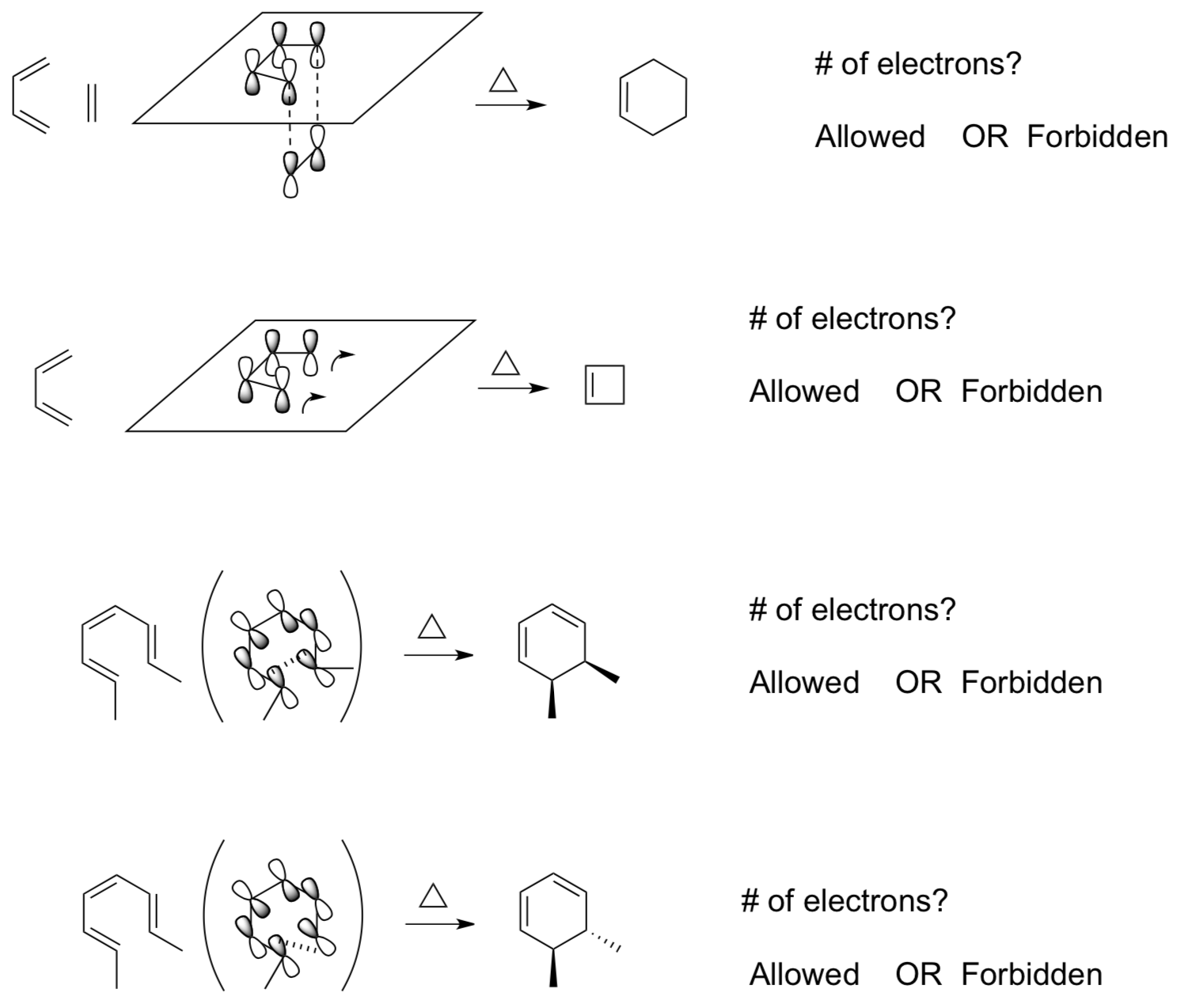
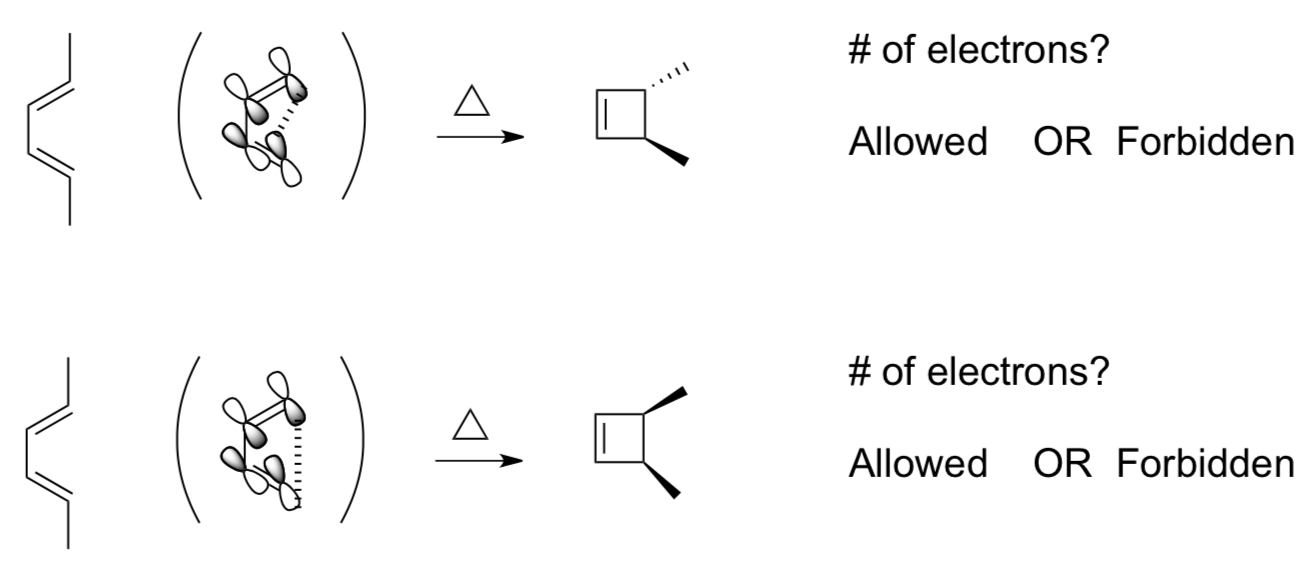
Simplified Woodward-Hoffman Rule #3 and #4
A pericyclic reaction involving 4n+2 electrons is photochemically allowed if there is an antarafacial interaction.
A pericyclic reaction involving 4n electrons is photochemically allowed if the orbital interact in a suprafacial manner.
In other words, the opposite rules apply under photochemical conditions!!
- Explain why reaction A is allowed under thermal conditions but reaction B is photochemically allowed.
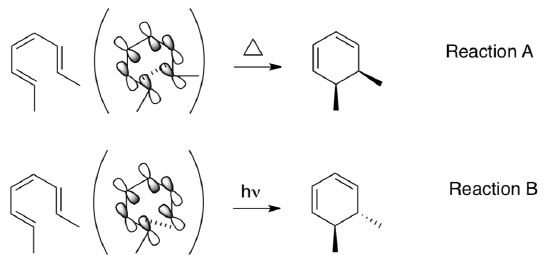
- Propose thermal or photolytic conditions for the following pairs of reactants.
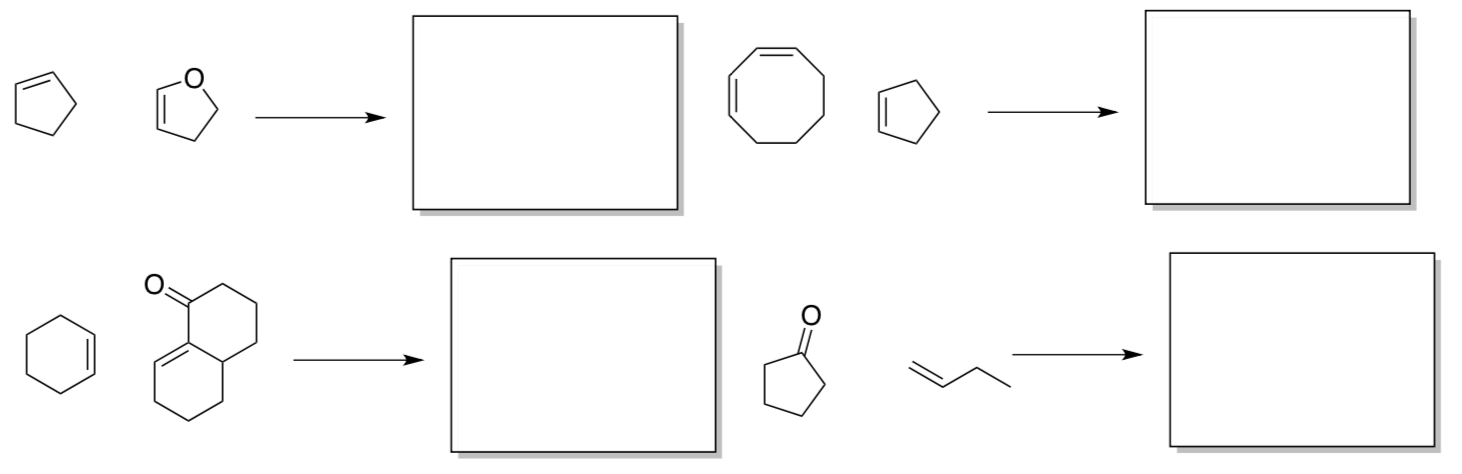
Summary of Cycloadditions
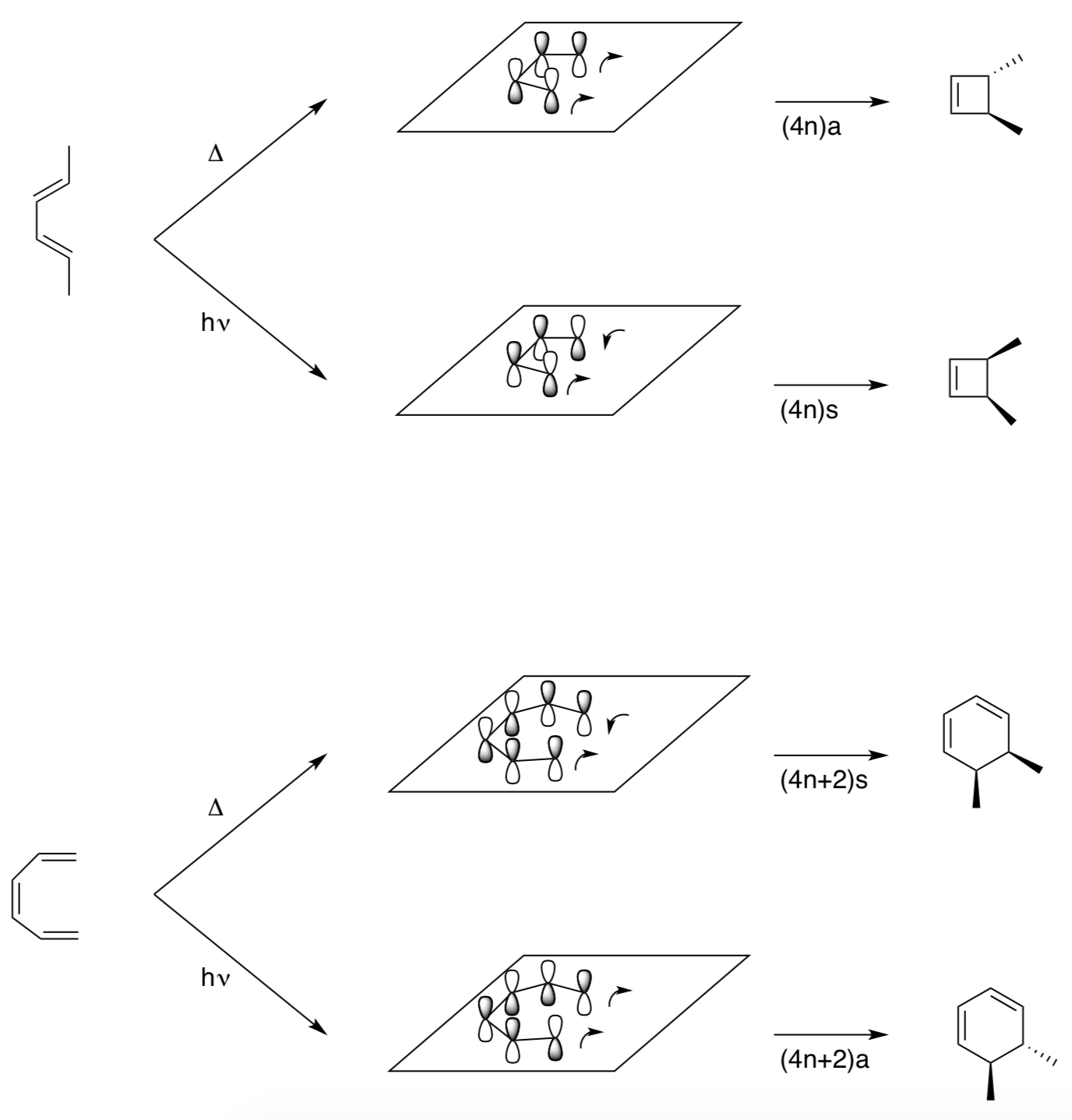
Summary of Electrocyclic Reactions
Practice Problems:
- Determine if each of these reactions would be allowed under thermal or photochemical conditions.
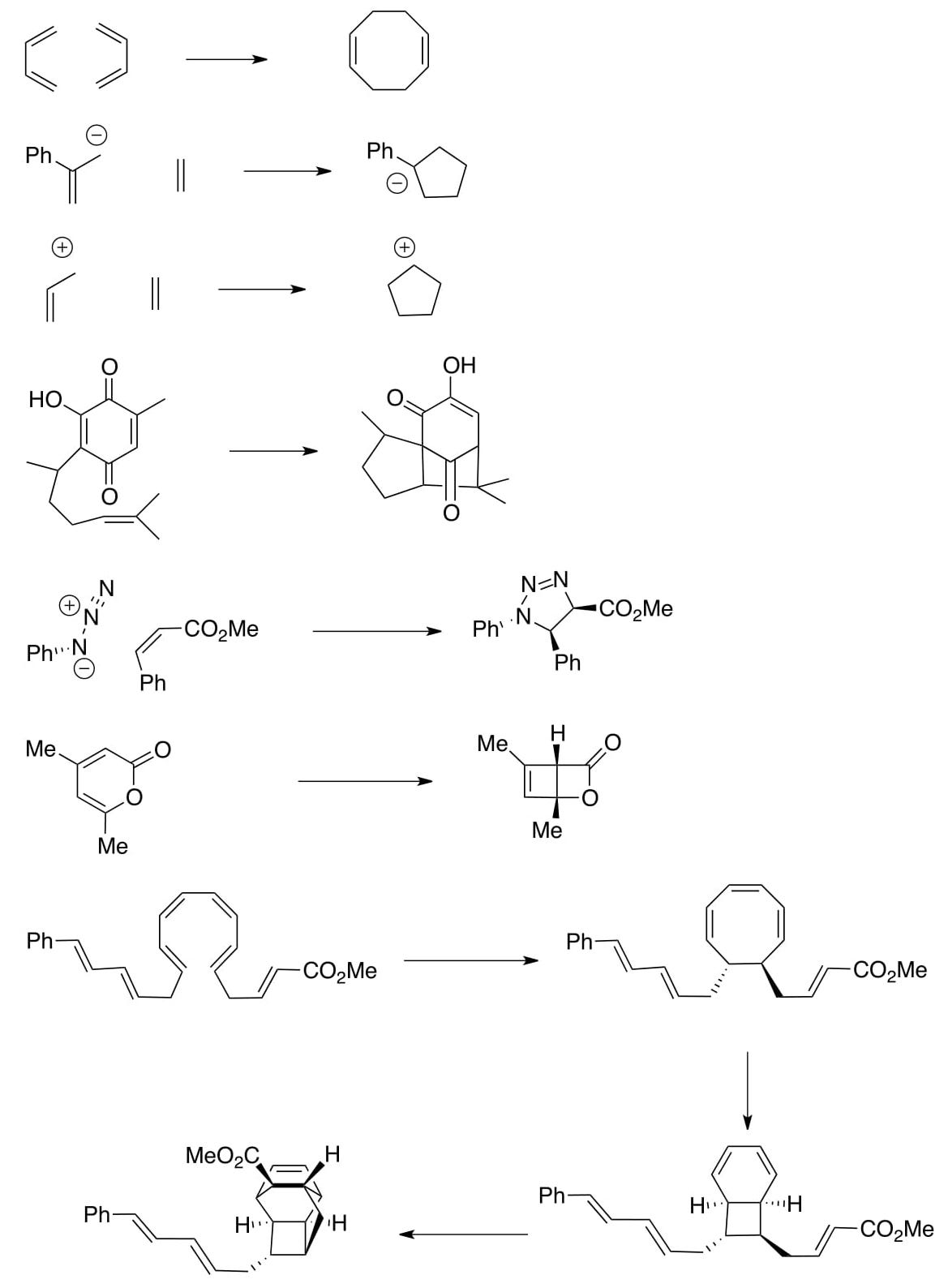
Application Problems:
- For the following electrocyclic reactions, draw out the HOMO (with correct shading of orbitals), and predict the product (make sure to include stereochemistry)
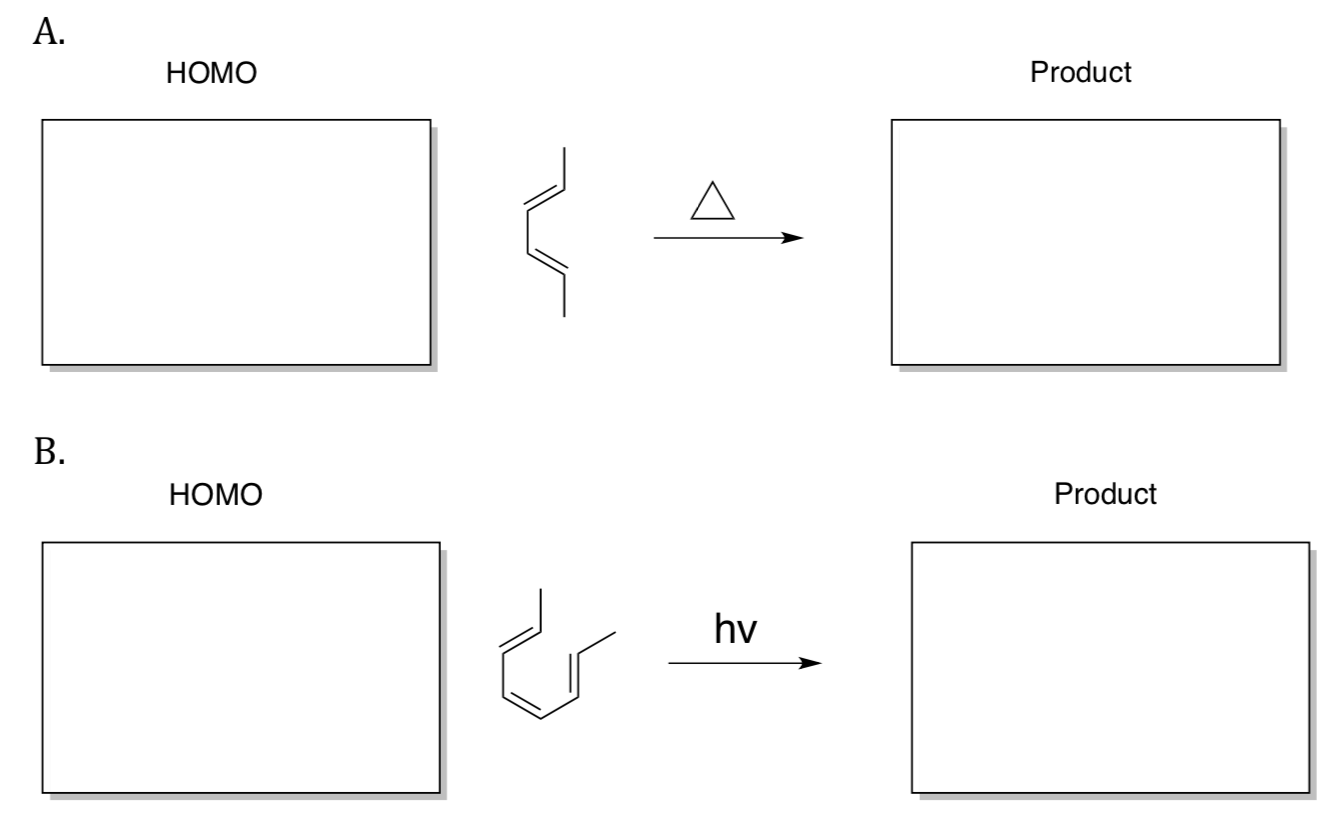
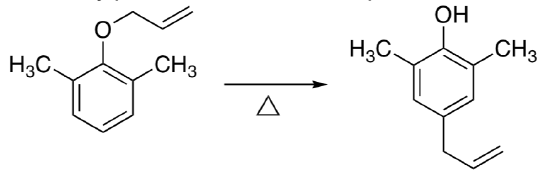
- When allyl 2,6-dimethylphenyl ether is heated 4-allyl-2,6-dimethylphenol is formed.
- Propose a mechanism for this reaction.
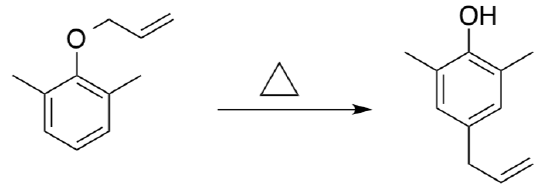
- Propose a mechanism for this reaction.
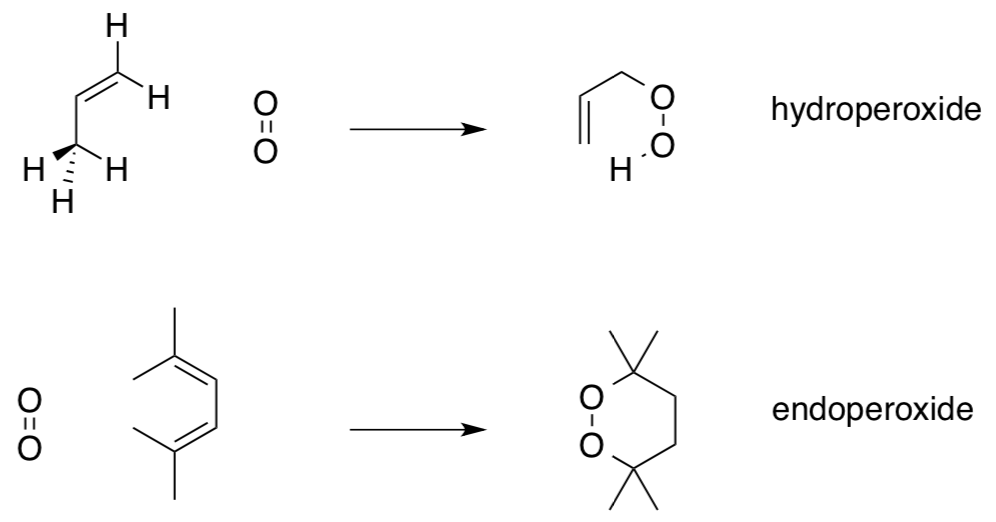
- Reaction with Singlet Oxygen
In contrast to triplet oxygen, singlet oxygen is kinetically more reactive.
- Why? (Hint: Draw the Lewis structure of singlet oxygen with all paired electrons.)
- Draw possible mechanisms for the follow reactions of singlet oxygen
- The α–hydroxycarbonyl moiety is an important structural motif in organic synthesis and is present in a substantial number of natural products. Nicholas Tomkinson recently reported several practical reagents for the preparation of α–oxygenated carbonyl compounds (Chem. Commun. 2005, 1478; Org Lett. 2005, 7, 5729).
- Predict the product of the reaction below.
- Draw the mechanism for the entire reaction sequence.
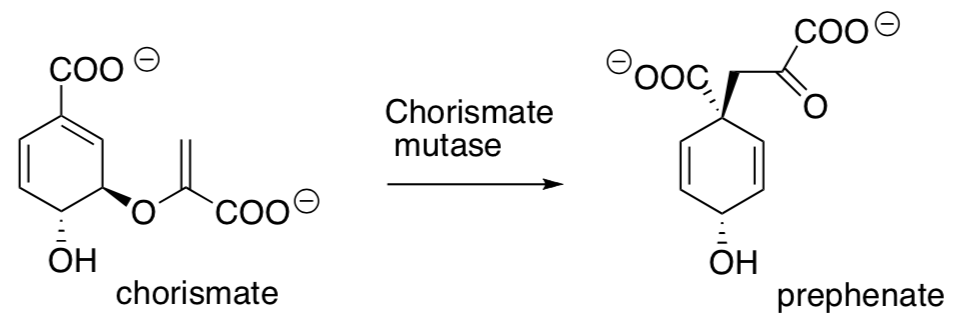
- Chorismate mutase is an enzyme that catalyzes the rearrangement of chorismate to prephenate in the Shikimic Acid Pathway. Propose a mechanism for this transformation.
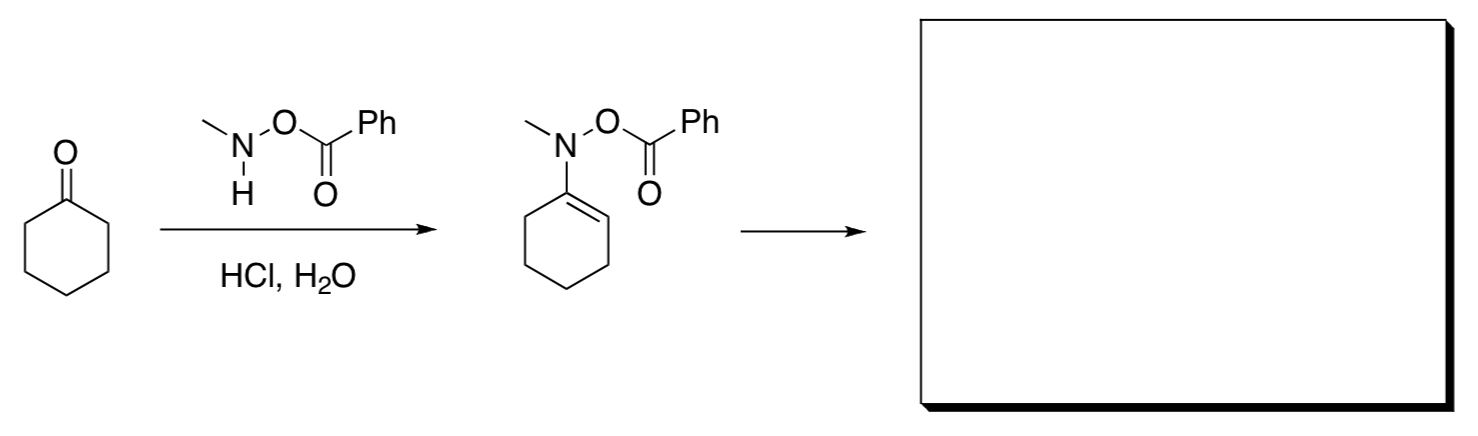
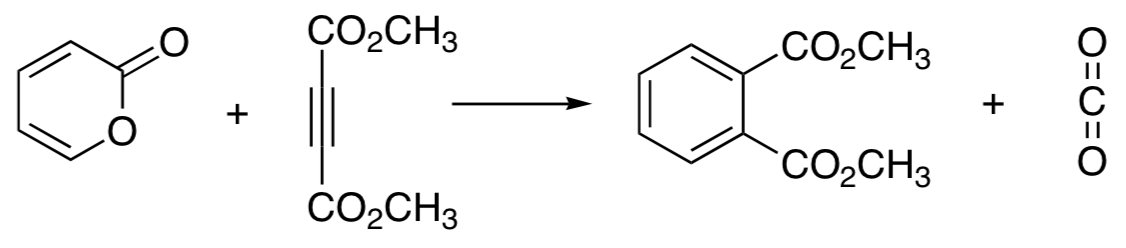
- This reaction has been shown to occur in two thermally allowed steps. Show the structure of the intermediate in the reaction and propose a mechanism. Each step is a name reaction. What are the name reactions involved?
- When heated, allyl phenyl ether undergoes a 3,3-sigmatropic rearrangement to form ortho-allylphenol.
- Propose a synthesis of allyl phenyl ether starting from phenol.
- Propose a mechanism for the rearrangement of allyl penyl ether to ortho- allylphenol.
- When allyl 2,6-dimethyphenyl ether is heated, a much different product, 4- allyl-2,6-dimethylphenol, is formed. Propose a mechanism for this reaction.
- Carbene Reactions (J. Am. Chem. Soc.,1973, 95, 8209).
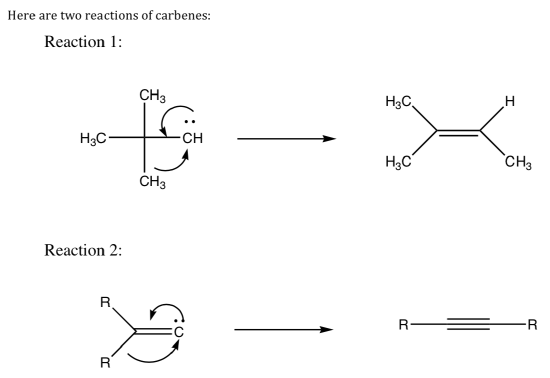
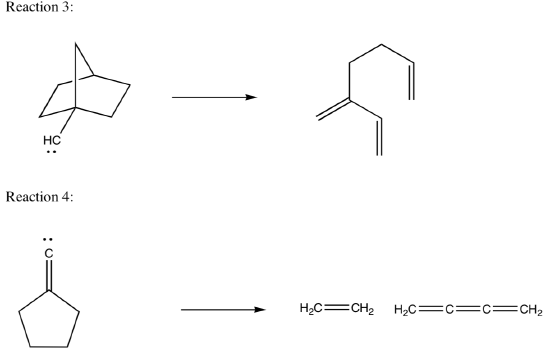
Now here are two analogous reactions that might have been expected to produce simple alkenes or alkynes but do not:
Your tasks:
- Draw the“expected”products of the two reactions.
- Why are the expected products not stable?
- Show a mechanism for formation of the real products.
- For Reaction 3, there are two possible routes to get the same products.
Draw both routes. Design an experiment to determine which route actually occurs.
Photocatalytic Cycloadditions
This exercise looks at the evolving approach of one laboratory to adapt a method of reactivity to a variety of systems.
Tehshik Yoon’s lab at the University of Wisconsin-Madison is interested in exploring the intersections of photochemistry, pericyclic reactions and radicals. Yoon was interested in developing 2+2 cycloadditions involving enones, below.
- Show the expected product of a 2+2 cycloaddition.
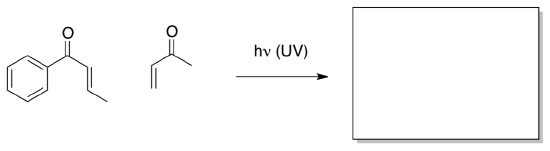
- Show the Huckel MO diagram for an enone on the left and a diagram for the excited state on the right. Draw the orbital cartoons, too.
- Circle the orbitals that would interact with each other in a 2+2 cycloaddition.
The products of the reaction under UV light are shown below.
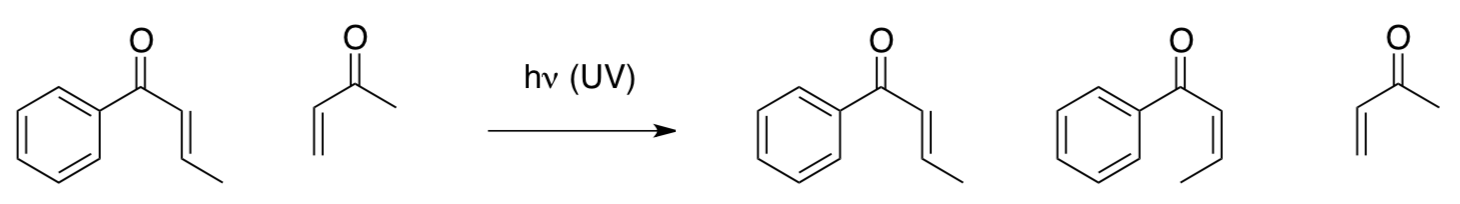
- Propose a reason the two molecules didn’t react together.
- Explain what did happen by discussing the Huckel MO diagram.
Photocatalytic Cycloaddition: 2+2 with Electron-poor Ene (JACS 2009)
- The problem is, these two enones are both electrophiles / nucleophiles (circle one).
- If one of them were to become an electrophile / nucleophile, (circle one) they might react together.
Yoon wanted to try a photocatalysis approach to this reaction. He decided to use bright red Ru(bpy)32+ as the catalyst. When a photon is absorbed, an electron is excited from the ruthenium to the bpy ligand.
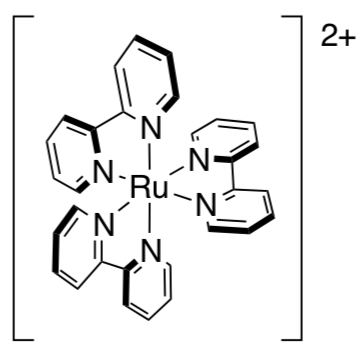
The resulting Ru3+ is reduced to Ru2+ by the amine. After relaxation from the bpy, the Ru+ delivers an electron to the enone on the left. After the 2+2 addition, an electron is transferred back to the amine.

- In this case, Ru(bpy)+ is acting as an oxidant / reductant (circle one).
- Draw a catalytic cycle for this sequence.
- Show the Huckel MO diagram of the nucleophilic enone as well as the electrophilic one and indicate which orbitals interact in the 2+2 addition.
- Usually photochemistry is done with high-intensity UV lamps. Comment on the significance of the light source in this case.
Photocatalytic Cycloaddition: 2+2 with Electron-rich Ene (JACS 2010)
This approach does not work with electron-rich alkenes. Instead, Yoon employed a catalytic amount of methyl viologen to accept an electron from the bpy-.
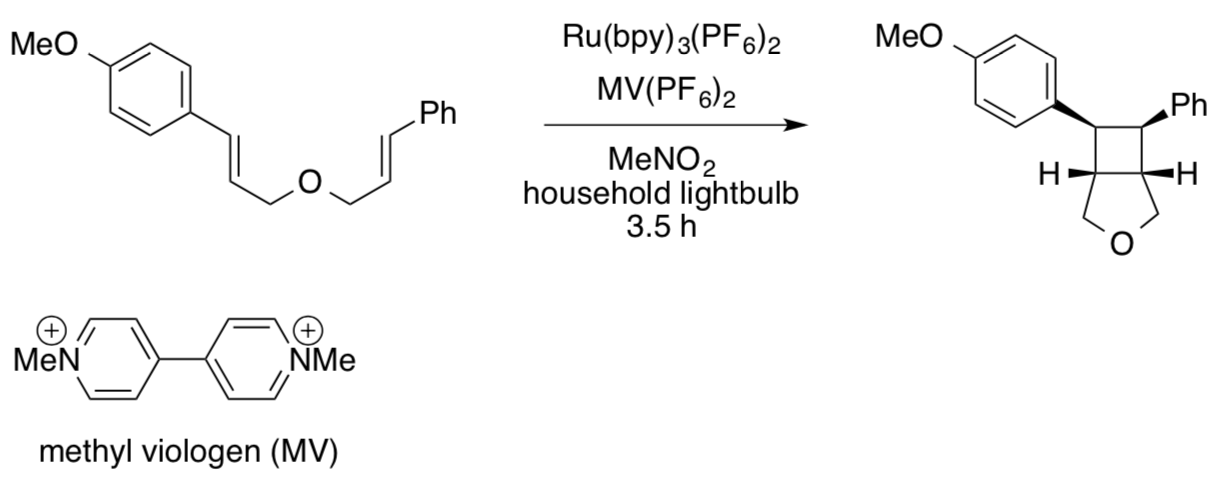
- In this case, Ru(bpy)3+ is acting as an oxidant / reductant (circle one).
- Provide a catalytic cycle for this reaction.
- Explain the comparative yields of cycloaddition products using the following derivatives. Resonance structures may help.

- Comment on the significance of the light source used in this case.
Photocatalytic Cycloaddition: Diels Alder with Electron-rich Ene (JACS 2011)
A similar approach was taken to catalyze Diels Alder reactions.
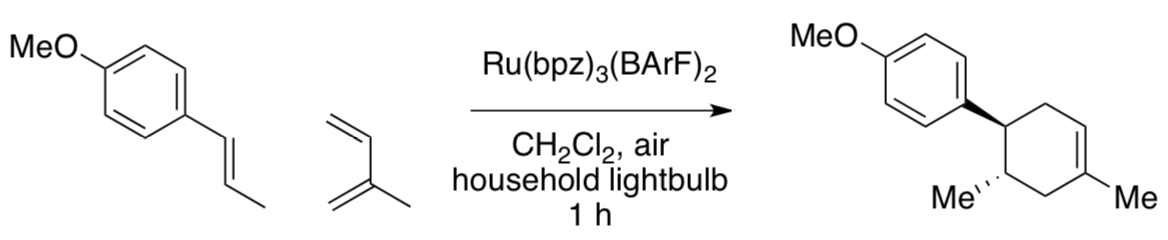
Note that a different catalyst was used (below). Redox potentials of some of the species involved are shown below.
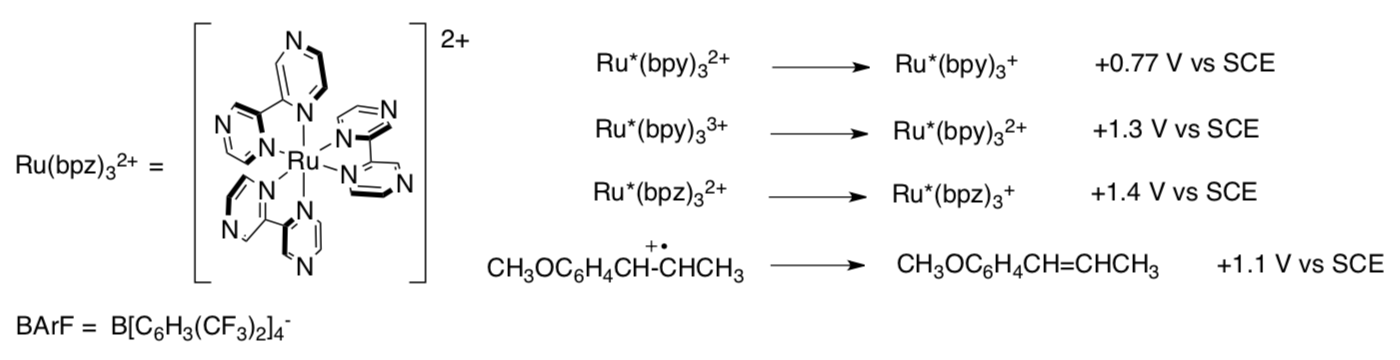
- Ru(bpz)32+ was used as an oxidant / reductant (circle one).
- Why did the Yoon lab use Ru(bpz)32+ instead of Ru(bpy)32+?
- They didn’t add MV2+ this time. Why not?
To complete a catalytic cycle, the Ru+ must be re-oxidized to Ru2+.
- What was used as the co-oxidant this time?
- Provide a catalytic cycle for the reaction.
Photocatalytic Cycloaddition: Application of Diels Alder
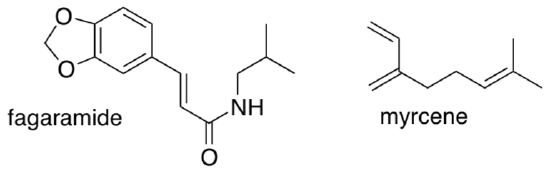
The Yoon lab applied their method in the synthesis of heitziamide A, a natural product isolated from the medicinal shrub Fagara heitzii.
- Fill in the missing products and reagents.
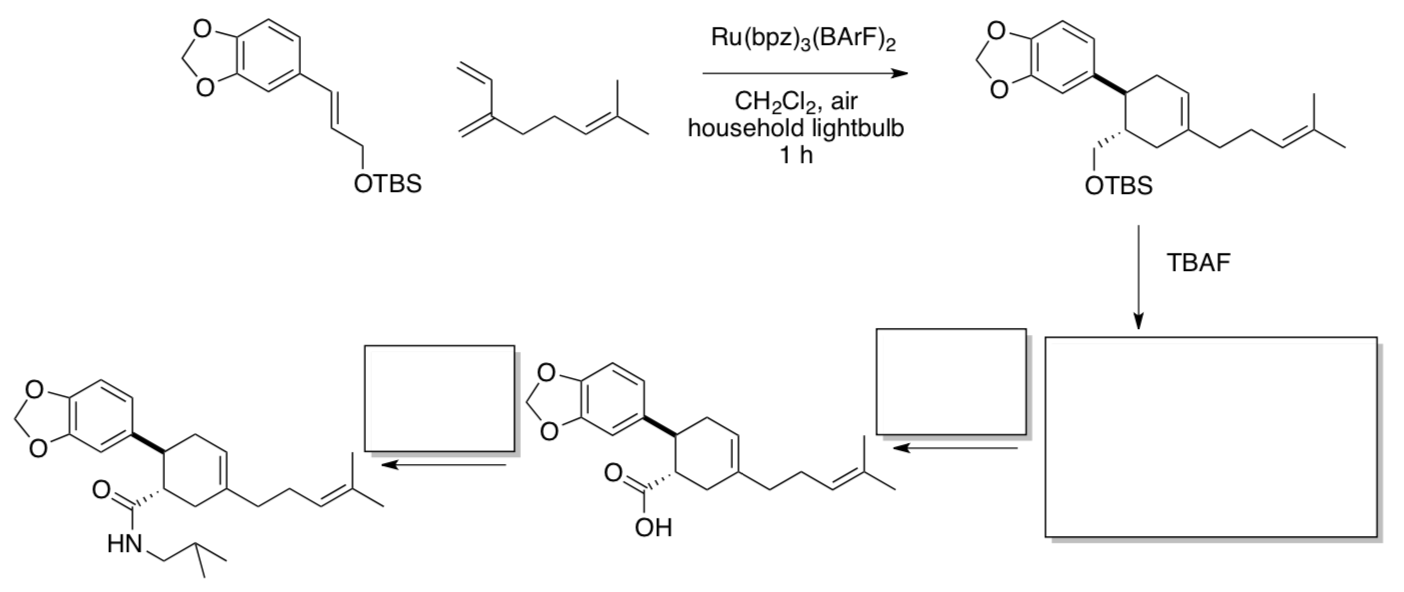
In contrast, a Diels Alder reaction carried out by heating for 72 h at 150oC provided the opposite regiochemistry.
- Show this alternate product.
Regiochemistry in Diels Alder arises from the interaction of the most electron rich end of the dienophile with the least electron rich ene of the diene (or vice versa).
- Show which end of the diene and dienophile are more electron-rich, and show how that changes under photocatalytic conditions.
- Myrcene and fagaramide are natural products of F. heitzii. Comment on the significance of this fact in terms of the biosynthesis of heitziamide A.
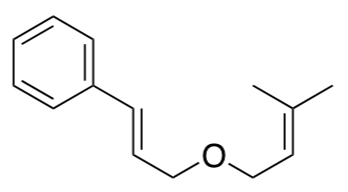
Photocatalytic Cycloaddition: 2+2 via Energy Transfer (Angew. Chem. IE 2012)
The styrene shown below has an oxidation potential of +1.42 V vs. SCE.
- Explain why that is a problem in terms of carrying out a 2+2 photocatalytic cycloaddition under conditions described previously.
The Yoon lab decided to turn to a new catalyst, shown below. It has an oxidation potential of +0.89 V vs SCE.

- Will this compound be more successful as an electron-transfer catalyst, based on this data?
The strategy this time was to transfer energy, rather than an electron, from the catalyst to the substrate. The excited iridium complex can form an excited state triplet (via “inter-system crossing”), the energy of which is about 60 kcal/mol above the ground state iridium complex.

That triplet can occasionally exchange energy with the styrene substrate, which also has a triplet state about 60 kcal/mol above its ground state. This process is called “photosensitization”. The excited styrene undergoes 2+2 cycloaddition.
- How much energy does the catalyst have left after transferring energy to the styrene?
- Describe the electronic state of the iridium after the transfer has occurred.
Photocatalytic Cycloaddition: 2+2 via Energy Transfer
- Would a similar strategy work with Ru(bpy)32+ or Ru(bpz)32+, which have triplet state energies of 46.8 and 47.1 kcal/mol, respectively? Why?
- Comment on the time required for this reaction, compared to previous ones. Why is it different?
A number of similar styrenes were subjected to these conditions; yields of the products are shown below.
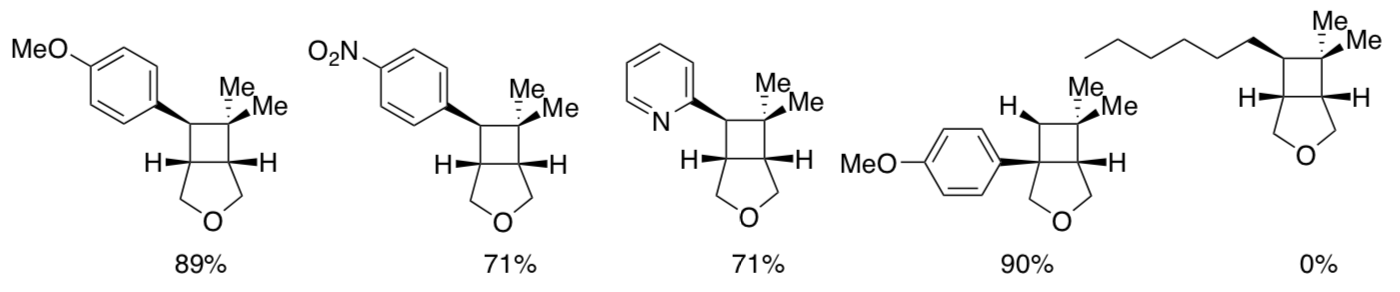
- Draw the substrate used in each case.
- What factors determined whether or not the reaction worked?
An application was carried out in the synthesis of cannabiorcycloic acid.
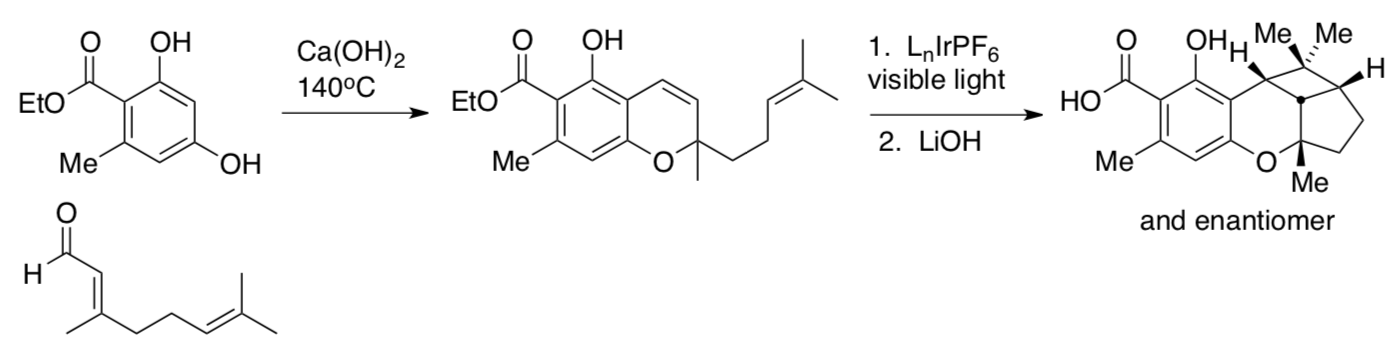
- Provide a mechanism for the first reaction in the synthesis.


