19b: Smog
- Page ID
- 150644
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\(\newcommand{\ket}[1]{\left| #1 \right>} \)
\( \newcommand{\bra}[1]{\left< #1 \right|} \)
\( \newcommand{\braket}[2]{\left< #1 \vphantom{#2} \right| \left. #2 \vphantom{#1} \right>} \)
\( \newcommand{\qmvec}[1]{\mathbf{\vec{#1}}} \)
\( \newcommand{\op}[1]{\hat{\mathbf{#1}}}\)
\( \newcommand{\expect}[1]{\langle #1 \rangle}\)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)Name: ______________________________
Section: _____________________________
Student ID#:__________________________
Atmospheric Chemistry: Photochemical Smog
Passport:
Under conditions where the atmosphere contains excessive amounts of oxidizable material, some of the intermediates can cause pollution problems such as photochemical smog.
Photochemical Smog
Photochemical smog is created in the troposphere.
- Identify this atmospheric layer.
Physical characteristic of smog include a brownish-yellow haze and the presences of compounds that irritate the eyes and respiratory tract.
- Use the internet to find out:
- Health effects of Photochemical Smog
Chemical make-up of Smog
Photochemical smog is usually made up of ozone, aldehydes, hydrocarbons, NO2 and organonitrates such as PAN (peroxyacetyl nitrate, CH2CO-O-O-NO2).
- Draw the Lewis structures for some of these compounds involved in smog formation:
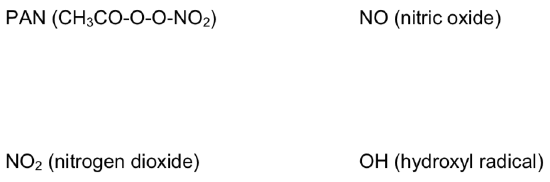
Formation of Photochemical Smog
There are several steps in photochemical smog formation. Nitrogen oxides are formed from a combustion reaction in the troposphere. In turn, these NOx radicals perpetuate the formation of a number of other radical species.
Reactions in the formation of smog:
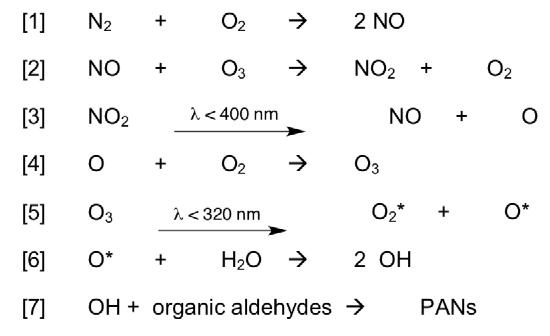
Reaction 1
Formation of nitric oxide (Rxn 1) is endothermic. The reverse reaction is very slow.
[1] N2 + O2 -> 2NO
- Draw a reaction profile diagram for this reaction. How big is the Ea?
NO is most commonly formed during lightning or combustion.
- Why does this reaction only form under conditions with higher temperatures?
- Use Le Chatelier’s Principle to explain what happens to the equilibrium concentration of NO as the temperature increases.
- When exhaust gases containing NO mix with cool air, you might expect that the NO will revert to N2 and O2. Explain why automobile exhaust can lead to a higher than equilibrium concentration of NO in urban areas.
Reaction 2
Ozone in troposphere??
[2] NO + O3 -> NO2 + O2
NO can catalytically destroy ozone without photochemical activation is required.
- Explain why this molecule (“as is”) can destroy ozone.
When the ratio of NO2 to NO is greater than 3, the formation of ozone is the dominant reaction. If the ratio is less than 0.3, then the nitric oxide reaction destroys the ozone at about the same rate as it is formed, keeping the ozone concentration below harmful levels.
Reaction 3
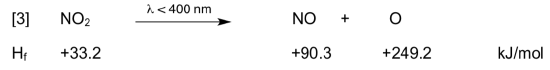
NO2 is photolyzed by UV radiation from the sun and decomposes into NO and an atomic oxygen (O). NO is a brown gas which is part of the color seen in smog.
- Use the enthalpies of formation to calculate ΔHrxn.
- Use this equation (ΔE = ΔH -ΔnRT) to calculate the ΔE required to break this bond.
Air quality is often measured as a sum of NO2 and NO concentrations called NOx.
- Why have environmental chemists chosen to measure the [NOx]?
Reaction 4
In the troposphere, nitrogen oxides engage in a complex range of reactions as outlined previously. Then atomic oxygen can react with diatomic oxygen (O2) to form toxic ground-level ozone.
Farrow, J. Phys. Chem. 1977, 6, 787.
- Using steady state approximation, assume the rate of change of atomic oxygen concentration is zero (rate of reactions involved in formation of O = rate of reactions consuming O). Show which reactions will be used.
- Determine the [O] in terms of other variables (ks and other concentrations).
- Using steady state approximation, assume the rate of change of ozone concentration is zero (rate of reactions involved in formation of O3 = rate of reactions consuming O3). Show which reactions will be used.
- Determine the [O3] in terms of other variables (ks and other concentrations).
- Substitute [O] from (b) into (d) to express ozone concentration in terms of other variables.
Reaction 5
The photochemical cleavage of ozone yields O2 and O. The O can be formed in either the ground state (O) or the excited state (O*).

Reaction 6. Formation of Hydroxyl Radicals

- Use the enthalpies of formation to calculate ΔHrxn.
- Is this reaction endothermic or exothermic?
- The Hf of O (ground state) is 249.2 kJ/mol. What is the ΔHrxn for the reaction of ground state O with H2O to form hydroxyl radicals?
- Is this reaction endothermic or exothermic?
Although reaction 6 has a large rate constant, only a small fraction of all excited oxygen atoms formed in reaction 5 actually go on to afford hydroxyl radicals.
- Suggest a reason why only a few of the O* from reaction 5 proceed to OH.
- How much of the hydroxyl radical will be formed at night?
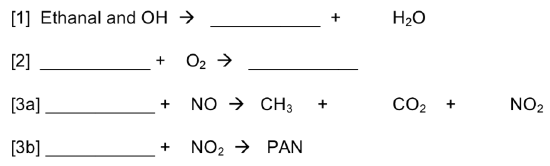
Reaction 7. Tropospheric oxidant to form PAN
Many of the components of smog are created when the hydroxyl radical oxidizes hydrocarbons in the atmosphere. OH radical is extremely reactive and typically exists for less than a second before undergoing a reaction.
- Propose a reaction of OH with NO2. (leading to acid rain)
- Propose a reaction of OH with methane.
- Propose a reaction of CH3 radical with O2.
- Propose a reaction of CH3-O-O-H with OH to form methanal.
- Write a balanced equation for the reaction of methanal with O2 to form CO.
- Complete the following reaction sequence to form PAN:
Smog Production throughout the Day
The reaction of hydrocarbons (unburnt petrol) with nitric oxide and oxygen produce nitrogen dioxide also in the presence of sunlight, increasing the ratio of nitrogen dioxide to nitric oxide.
A profile of the concentrations of the pollutants in photochemical smog, and how they vary throughout a day are shown below:
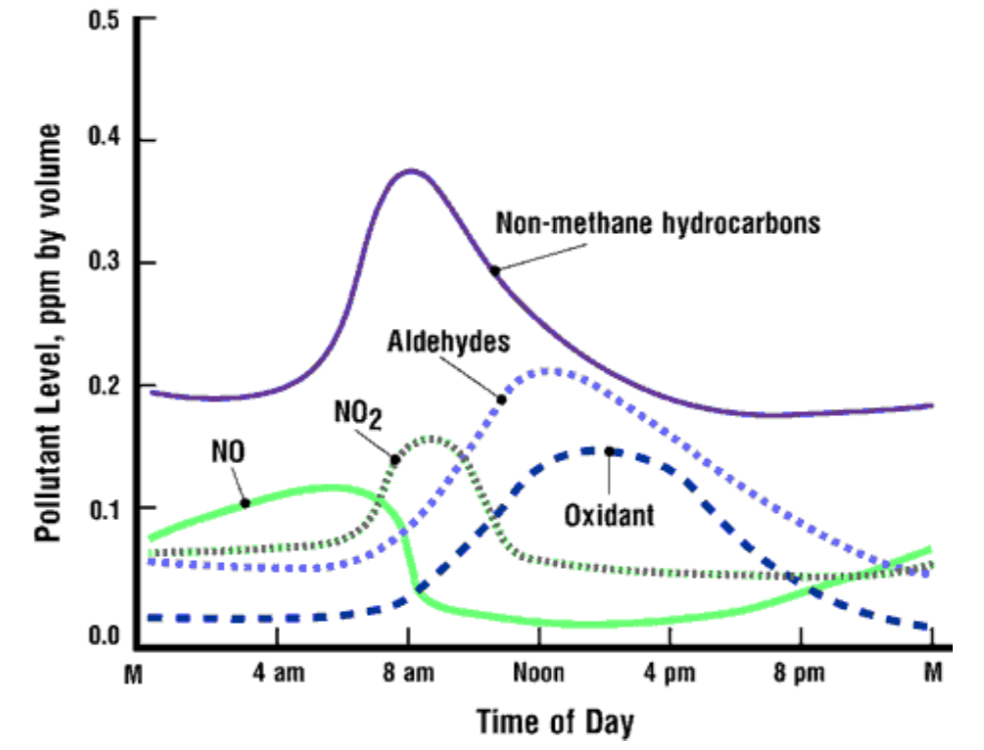
- Why does the atmospheric concentration of non-methane hydrocarbons peak at 8:00 a.m., and then decrease throughout the day?
- Why does the atmospheric concentration of aldehydes peak at 1:00 p.m., and then decrease throughout the afternoon?
- Why does the atmospheric concentration of oxidants (PAN) peak at 3:00 p.m.?
- How can pumping your gas at night reduce photochemical smog formation?
- Why is it not a good idea to breathe ozone?
Application:
Ozone is a normal trace component of the troposphere but its concentration may rise under conditions of severe pollution.
- In the following locations, would you expect to encounter abnormally high levels of tropospheric ozone?
- Houston, TX in May:
- Canadian Prairies in August:
- New York, NY in February:
Remediation of Smog
Catalytic converters on motor vehicle exhausts are a way of trying to reduce the carbon monoxide and nitrogen oxide emissions.
A catalytic converter uses the basic redox reactions to help reduce the pollutants a car makes. It converts around 98% of the harmful fumes produced by a car engine into less harmful gases.
A three-way catalytic converter has three simultaneous functions:
- Reduction of nitrogen oxides into elemental nitrogen and oxygen.
NOx → Nx + Ox
- Oxidation of carbon monoxide to carbon dioxide.
CO + O2 → CO2
- Oxidation of hydrocarbons into carbon dioxide and water.
CxH4x + 2xO2 → xCO2 + 2xH2O
The catalyst used is combination of platinum and rhodium.
The platinum catalyzes the reaction of unburnt hydrocarbon (such as pentane) and oxygen (O2) to produce carbon dioxide (CO2) and water vapour (H2O).
- Balance this equation.
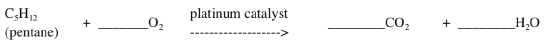
The rhodium catalyzes the reaction of carbon monoxide (CO) and nitric oxide (NO) to form carbon dioxide (CO2) and nitrogen gas (N2):
- Balance this equation.



